Published TCIMAIL newest issue No.197 | New tool "TCI SpectraViewer" | [Product Highlights] o-Nitrobenzyl Photolabile... | Product Document Searching Made Easy by 2D Code!
Maximum quantity allowed is 999
Please select the quantity
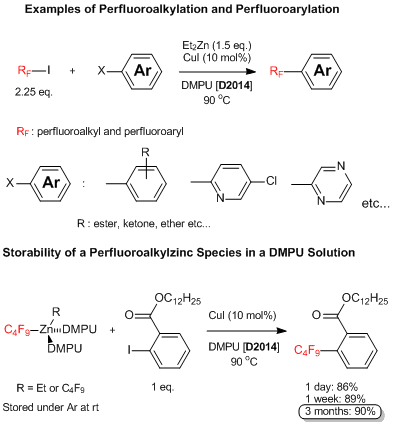
Cross-coupling Reactions of Perfluoroalkyl Zinc Reagents from Perfluoroalkyl Halides
Uchiyama et al. have reported that perfluoroalkyl zinc reagents are able to be prepared from perfluoroalkyl halides like perfluorobutyl iodide and diethylzinc and can be used for cross-coupling reactions in the presence of a copper salt. In this reaction, various aryl and heteroaryl halides can react with perfluoroalkylzinc species and the desired perfluoroalkyl group-substituted aromatic rings are given. A noteworthy fact is that the perfluoroalkylzinc reagent prepared in a DMPU solvent is extremely stable at room temperature under argon atmosphere even after being stored for three months; it shows high reactivity without a decrease in yield. This synthetic method is generally useful because easily available perfluoroalkyl or perfluoroaryl halides can be treated for the preparation of perfluoroalkyl/perfluoroaryl zinc species. Recent attention has been paid to fluorocarbons, so, this reaction is expected to be applied to the study of pharmaceuticals and electronic materials.