Maximum quantity allowed is 999
Please select the quantity
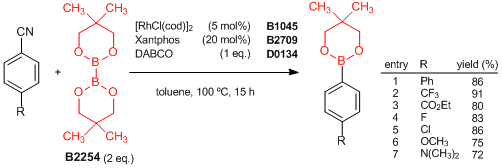
Rhodium(I)-Catalyzed Aryl Nitrile Borylation through the Cleavage of Carbon-Cyano Bonds
Tobisu et al. have reported a rhodium(I)-catalyzed borylation of aryl nitriles using bis(neopentyl glycolato)diboron. The reaction of aryl nitriles with the diboron in the presence of the rhodium/Xantphos catalyst and DABCO affords these arylboronic esters, even when there are other functional groups (R), via carbon-cyano bond cleavage. The carbon-cyano bond is not cleaved under typical transition-metal-catalyzed conditions, which allows the regioselective coupling to be combined with other transition-metal-catalyzed couplings. Therefore, this reaction would be a new strategy for the synthesis of complex boronic acid derivatives.