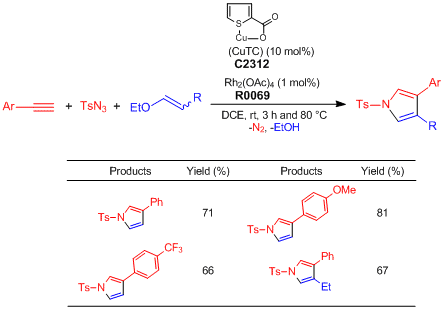
Application
One-pot Synthesis of Pyrroles from Terminal Alkynes, N-Sulfonyl Azides, and Alkenyl Alkyl Ethers
Typical Procedure:
Alkyne (0.2 mmol), TsN3 (1.0 eq.), alkenyl alkyl ether (3.0 eq.), CuTC (10.0 mol%), Rh2(OAc)4 (1.0 mol%), and DCE (0.2 M) are added to an oven-dried test tube under nitrogen atmosphere. The reaction mixture is stirred at ambient temperature for 3 h and then, heated at 80 °C for 4–13 h. After the reaction mixture is filtered through short path of celite with CH2Cl2, the filtrate is concentrated under reduced pressure. The residue is purified by silica gel column chromatography (ethyl acetate–hexane) to give pyrrole derivatives (Y. 65–81%).
References
Application
Highly Diastereoselective Synthesis of Fully Substituted Tetrahydrofurans
Typical Procedure: A flask is charged with allyl alcohol (0.10 mmol, 1.0 eq.), Rh2(OAc)4 (2.0 mol%) and 4A MS (0.1 g) in toluene (1.5 mL), the reaction mixture is stirred at 45℃, and a solution of the diazo compound (0.20 mmol) in toluene (0.5 mL) is added to the reaction mixture over a period of 1 h through a syringe pump. After completion of the addition, piperidine (2.0 eq.) is added and the reaction mixture is stirred for additional 5-10 h (reaction completion is monitored by TLC) at 45℃. The reaction mixture is purified by flash chromatography on silica gel (EtOAc : light petroleum ether = 1 : 50 to 1 : 20) to give the pure product.
References
PubMed Literature