Application
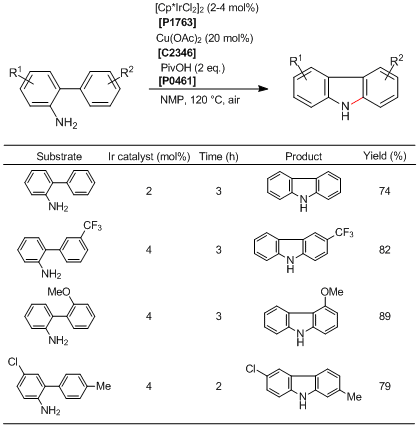
Synthesis of N―H Carbazoles via Ir-catalyzed Intramolecular C―H Amination
Typical Procedure:
To a 20 mL two-necked flask with a reflux condenser and a rubber cap are added 2-aminobiphenyls (0.5 mmol), [Cp*IrCl2]2 (0.01 mmol, 8.0 mg), Cu(OAc)2 (0.1 mmol, 18 mg), pivalic acid (1 mmol, 102 mg), 1-methylnaphthalene (ca. 30 mg) as an internal standard, and N-methyl-2-pyrrolidone (NMP, 3 mL). Then, the resulting mixture is stirred under air at 120 °C for 3-6 h. After cooling, the reaction mixture is extracted with ethyl acetate (100 mL). The organic layer is washed by aqueous NaHCO3 (100 mL×3), and dried over Na2SO4. After evaporation of the solvents under vacuum, the product is isolated by column chromatography on silica gel using hexane-ethyl acetate (10:1) as the eluent.
References
Application
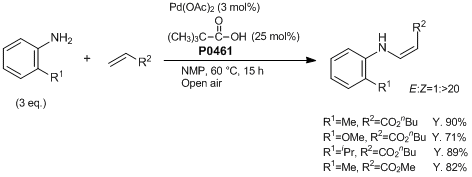
Pd-Catalyzed Z-Selective Oxidative Amination of Olefins
Typical Procedure:
A mixture of butyl acrylate (128 mg, 1.0 mmol) and o-toluidine (322 mg, 3.0 mmol) is allowed to react with Pd(OAc)2 (6.7 mg, 0.03 mmol, 3 mol%) and pivalic acid (26 mg, 0.25 mmol) in NMP (0.5 mL) at 60 °C for 15 h under open air in a 30 mL round-bottomed flask. The crude reaction mixture is cooled to room temperature. EtOAc (30 mL) is added to the dark solution. The residue is purified by column chromatography on silica gel (EtOAc : Hexane = 1 : 40) to provide (Z)-butyl 3-(o-tolylamino)acrylate (210 mg, Y. 90%).
References
PubMed Literature