Application
2-PySO2CF2H : Bench-stable Difluoromethylating Agent for Catalytic Difluoromethylation
Reference
- Iron-Catalyzed Di?uoromethylation of Arylzincs with Di?uoromethyl 2?Pyridyl Sulfone
Application
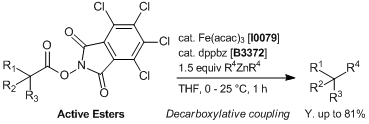
Iron/dppbz-catalyzed Decarboxylative Couplings of Active Esters
In a screwed-capped vial active ester (0.1 mmol), Fe(acac)
3 (0.005 – 0.04 mmol) and
dppbz (0.006 – 0.048 mmol) are weighed. The vial is then sealed, evacuated and back-filled with Ar. Then, 0.5 mL of distilled THF is added. The mixture is stirred for 5 minutes under Ar and diarylzinc reagent in THF (
ca. 0.3 mol/L, 0.15 – 0.25 mmol) is added in one portion at 0 - 25 °C to the mixture and stirred at the same temperature for 1 h. After this time, the reaction is quenched with 1 mol/L HCl
aq, sat. NH
4Cl
aq or H
2O and diluted with diethyl ether. The organic layer is separated and dried over Na
2SO
4 anhydrous, filtered and evaporated to dryness. Pure products are obtained after column chromatography or preparative TLC.
References
Application
Iron-Catalyzed ortho-Alkylation of Carboxamides

Typical procedure (R = benzyl): To Fe(acac)3 (17.1 mg, 0.05 mmol), dppe (29.9 mg, 0.075 mmol), and 3-methyl-N-(quinolin-8-yl)benzamide (131 mg, 0.5 mmol) are added THF (4 mL), and benzyl chloride (190 mg, 1.5 mmol). The mixture is then placed in an oil bath at 65 °C and PhMgBr (16% in THF, ca. 1 mol/L) (0.6 mL, 0.6 mmol) is added in a single portion. Upon completion of PhMgBr addition, the reaction mixture is stirred at 65 °C for 1 min. Then PhMgBr (16% in THF, ca. 1 mol/L) (1.3 mL, 1.3 mmol) is added dropwise at 65 °C for 9 min. The reaction mixture is stirred at 65 °C for 1 min and brought to rt. Subsequently the reaction mixture is quenched with a saturated solution of ammonium chloride (535 mg, 0.01 mol) and extracted with dichloromethane (3 x 3 mL). The combined organic extracts are dried over MgSO4, filtered and concentrated to give a residue. The residue is purified by silica gel flash chromatography (eluent: hexanes/EtOAc = 20:1) to give 2-benzyl-5-methyl-N-(quinolin-8-yl)benzamide (175 mg, 99% yield) as a white solid.
References
Application
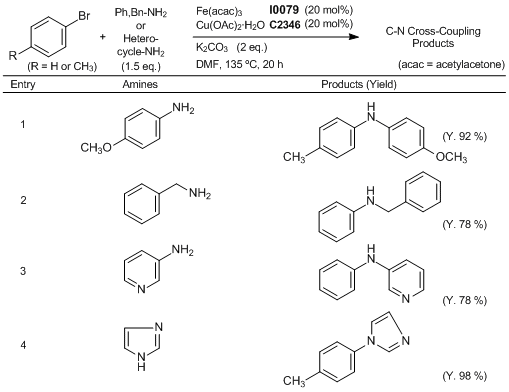
Efficient Fe/Cu Co-Catalyzed C-N Cross-Coupling Reaction
Typical procedure (Entry 1): A Schlenk tube is charged with p-anisidine (92.4 mg), K2CO3 (138 mg), Fe(acac)3 (34.4 mg), and Cu(OAc)2· H2O (20 mg). Under a nitrogen atmosphere, 4-bromotoluene (85.5 mg) is added followed by dry DMF (0.8 mL). The reaction mixture is then heated to 135 °C, and stirred for 20 h. The mixture is cooled to room temperature and diluted with diethyl ether. The resulting suspension is filtered through a celite pad and the filtrate is concentrated. The residue is purified by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate) to give 4-methoxy-N-p-tolylaniline (98.1 mg, 92 %).
References
Application
Iron-Catalyzed Cross-Coupling Reaction
Reference
PubMed Literature