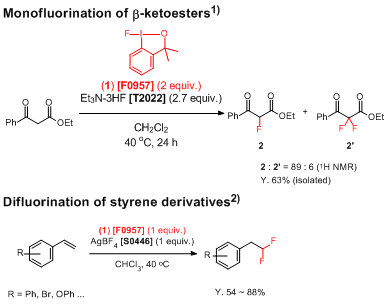
Monofluorination of β-ketoesters
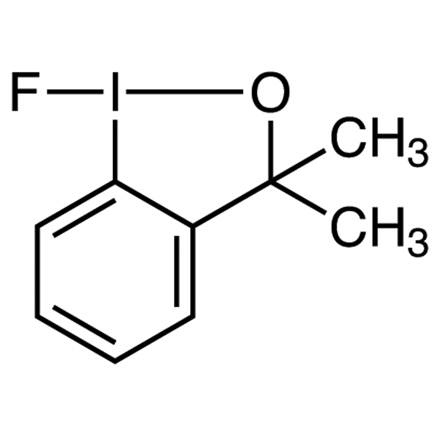
1) : Ethyl 3-oxo-3-phenylpropanoate (0.062 mL, 0.357 mmol) is added to a solution of 1-fluoro-3,3-dimethyl-1,2-benziodoxole
1 (0.200 g, 0.715 mmol) in
Et3N・3HF (0.71 M solution in dichloromethane, 1.4 mL, 0.96 mmol). The flask is then sealed and heated to 40 °C for 24 hours. After the reaction, the reaction mixture is cooled to room temperature and a saturated solution of NaHCO
3 (4 mL) is added. The organic layer is separated and the aqueous layer is extracted with more dichloromethane (3 x 5 mL). The organic layers are then combined, dried and concentrated. The crude product is purified by column chromatography (silica gel, eluent: EtOAc/Hexane = 5/95) to provide
2 as a yellow oil (0.106 g, 0.225 mmol, 63%).
3-Ethoxycarbonylbenzenediazonium tetrafluoroborate (132.0 mg, 0.5 mmol), 1-fluoro-3,3-dimethyl-1,2-benziodoxole (28.0 mg, 0.1 mmol) and a solution of
BF3·OEt2 (0.1 mmol) in benzotrifluoride (5.0 mL) are charged into flask. The mixture is sealed. Stirred for 36 h at 45 °C, the mixture is directly purified through column chromatography on silica gel with petroleum ether : diethyl ether = 40:1 as the eluent to give ethyl 3-fluorobenzoate as a colorless oil (58.2 mg, 70%).