Application
Carbon-Heteroatom Bond Formation at the Benzylic Position of Methylarenes
Typical procedure (Ar = 4-BrPh, nucleophile = PhCO2H): To a solution of 4-bromotoluene (342.1 mg, 2.0 mmol) in anhydrous MeCN (4 mL) are added 1,3-dibromo-5,5-dimethylhydantoin (314.5 mg, 1.1 mmol) and AIBN (16.8 mg, 0.1 mmol) at room temperature. The mixture is stirred for 2 h at 90 °C. Then, benzoic acid (268.7 mg, 2.2 mmol) and DIPEA (0.35 mL, 2.0 mmol) are added at room temperature and the mixture is stirred for 16 h at 90 °C. The mixture is quenched by the addition of saturated aq. Na2SO3 solution (10 mL) and then extracted with CHCl3 (3 × 20 mL). The organic layer is dried over Na2SO4. After removal of the solvent under reduced pressure, the residue is purified by column chromatography on silica gel (eluent: hexane/CHCl3 = 1/1) to afford 4-bromobenzyl benzoate as a white solid. (489.1 mg, 84% yield).
References
Application
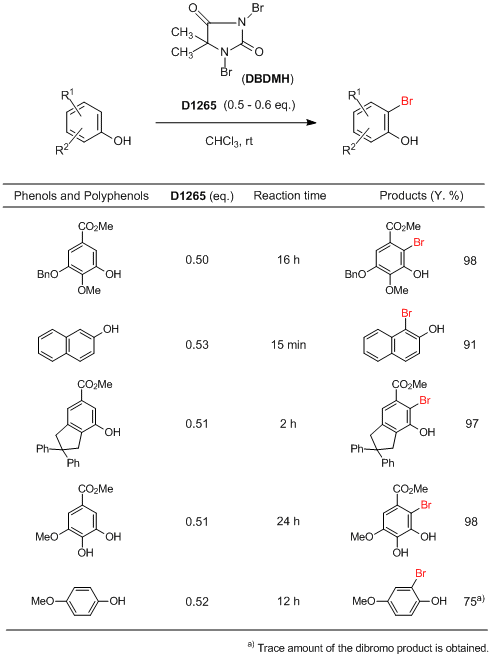
Selective ortho-Monobromination of Phenols and Polyphenols
Typical Procedure:
DBDMH (0.50–0.53 eq.) is added in part into the solution of starting material in CHCl3 (5–7 mL / mmol) at room temperature. Upon addition of the DBDMH, the solution becomes red or deep brown colored, the next portion of DBDMH is added after the disappearance of color and so on. The progress of the reaction is monitored by GC-MS. After completion of the reaction, removal of the solvent followed by the separation of the solid byproduct (derived from DBDMH) by simple filtration provides the almost pure bromide. 10% Aqueous sodium hydrosulfite solution is added into the reaction mixture, stirred for 5 min. Organic layer is separated, dried over MgSO4 and concentrated in evaporator yields the almost pure product. Passing through a small chromatographic column, it gives the pure products.
References
- 1,3-Dibromo-5,5-dimethylhydantoin, a useful reagent for ortho-monobromination of phenols and polyphenols
- A. Alam, Y. Takaguchi, S. Tsuboi, J. Fac. Environ. Sci. Tech., Okayama Univ. 2005, 10, 105.
PubMed Literature