Published TCIMAIL newest issue No.198
Maximum quantity allowed is 999
Please select the quantity
Reagent for the Formation of Borylcyclopropanes
No.185(December 2020)
2-(Diiodomethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (1) is a reagent for the synthesis of multisubstituted cyclopropane rings. Charette’s group reported the generation of carbenoids from 1 with ethylzinc trifluoroacetate and further transformation through the Simmons-Smith reaction.1) In addition, Takai et al. reported the borylcyclopropanation of unactivated alkenes with 1 in the presence of chromium(II) chloride and TMEDA.2) The obtained borylcyclopropanes can be further functionalized from the boronic ester moiety.
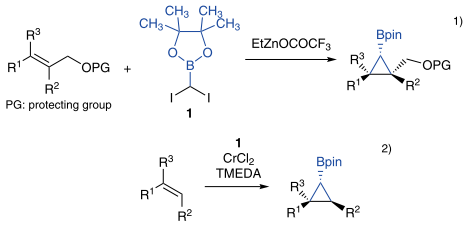
References
- 1) Diastereoselective borocyclopropanation of allylic ethers using a boromethylzinc carbenoid
- 2) Synthesis of borylcyclopropanes by chromium-promoted cyclopropanation of unactivated alkenes
Related Compounds
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
