Haruhiko Taguchi
Tokyo Chemical Industry, Co. Ltd.
About 22,000 reagents are available from TCI. It is considered that each of them has various usages for its research field and almost all of them would be used as major usages such as synthetic reagents, building blocks, electronic materials, life science materials and so on. On the other hand, a number of reagents have been used in a minor but unique synthetic manner. In this column, we will present another usage of reagents from the viewpoint of the reagent company.
In this issue, I pick Tebbe and Petasis reagents. It is well known that Tebbe and Petasis reagents are methylenation reagents of carbonyl compounds and they are available from TCI. Most users actually buy these reagents for such a usage. We can see by the strategies in many articles of organic synthesis that methylenation using either one and following with Grubbs reagent promoted ring-closing metathesis (RCM) is very efficient in total synthesis. I’m sure that Tebbe and Petasis, and of course, Grubbs reagents are essential reagents for synthesis of macrocyclic compounds because terminal olefins can be easily obtained from Tebbe or Petasis reagents.

We had performed macrocyclizations for the synthesis of a large number of cyclic compounds by Yamaguchi-macrolactonization and Corey-Nicolaou macrolactonization. And then, the intramolecular Mizorogi-Heck reaction had been developed and applied for these syntheses. In the 90’s, Ru- and Mo-promoted RCM had been developed, and now this synthetic procedure has been one of the most useful methods for the synthesis of the large cyclic compounds.1)
Meanwhile, carbene complexes formed from Ru or Mo metals have been well used for RCM because these carbene complexes have high affinities and reactivities for terminal olefins especially. While Tebbe and Petasis reagents are the precursors of Ti-carbene complexes, in situ generated Ti-methylidene complexes react with olefins to proceed to olefin metathesis with similarities to Ru- or Mo-carbene complex-promoted olefin metathesis.
However, in the case of Ti-carbene complexes, the reactivity is different from other metal-carbene complexes. Ti-carbene complex shows a higher affinity for the carbonyl group compared with the olefin moiety and this result impresses us as this reagent can be used for carbonyl olefination. Actually, Tebbe and Petasis reagents are the reagent for carbonyl olefination, but they can be also used for olefin metathesis. I considered how can use these reagent for olefin metathesis similar to Grubbs reagent and were there some chemists who had similar idea? So I searched related references and found that K. C. Nicolaou and his coworkers studied the reactivities of RCM using Tebbe and Petasis reagents.2) I will summarize the contents of these articles.
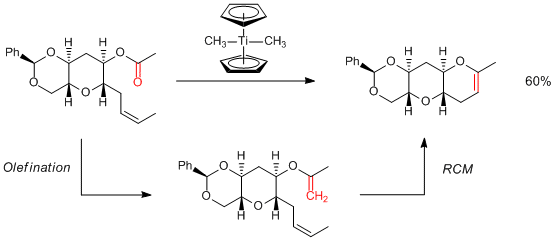
Nicolaou et al. studied RCM of marine natural products having a cyclic polyether structure. As a result, 6- and 7-membered cyclic polyethers were successfully synthesized by using Tebbe or Petasis reagents. However, this synthetic method was effective only when fixed-structure substances such as cyclic polyethers were employed. No desired products were afforded when substances having high flexibility such as acyclic polyethers were used. These results impress on us that the Grubbs reagent is the great reagent which can be used for various substances in RCM.
Recently, RCM using CH3CHBr2-TiCl4-Zn system has been reported.3) This reagent proceeds formally with the same reaction as other titanium reagents and even more, it shows high reactivity against Tebbe and Petasis reagents. This is the first report that acyclic substances of a diene can be used for titanium mediated RCM. Organotitanium reagents have been developed step by step and they may be used as one of the methods for macrocyclization in the future.
As described above, Tebbe and Petasis reagents are a powerful tool for methylenation of carbonyl compounds and they have another usage for RCM. Though this usage is limited for substances, I think these reagents are attractive and unique reagents. You can choose various organotitanium reagents from TCI for the study of organic synthesis.
References
- 1) K. C. Nicolaou, P. G. Bulger, D. Sarlah, Angew. Chem. Int. Ed. 2005, 44, 4490.

- 2) a) K. C. Nicolaou, M. H. D. Postema, C. F. Claiborne, J. Am. Chem. Soc. 1996, 118, 1565.
 b) K. C. Nicolaou, M. H. D. Postema, E. W. Yue, A. Nadin, J. Am. Chem. Soc. 1996, 118, 10335.
b) K. C. Nicolaou, M. H. D. Postema, E. W. Yue, A. Nadin, J. Am. Chem. Soc. 1996, 118, 10335.
- 3) K. Iyer, J. D. Rainier, J. Am. Chem. Soc. 2007, 129, 12604.


