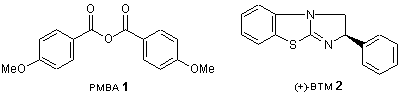The Kinetic Resolution of Racemic Alcohols and Racemic Carboxylic Acids
Recently, Shiina and co-workers developed the kinetic resolution of racemic alcohols and racemic carboxylic acids using 4-methoxybenzoic anhydride (PMBA, 1), and a Birman-type asymmetric catalyst1) [(+)-benzotetramisole, (+)-BTM; 2].2) For example, in the reaction of racemic alcohols with achiral carboxylic acids, first the mixed anhydrides are formed from 1 and the carboxylic acids. Subsequently the mixed anhydrides and one enantiomer of the racemic alcohols react preferentially to form optically active esters and optically active alcohols using catalyst (+)-BTM.
If this reaction is performed replacing the reactive substrates with racemic carboxylic acids and achiral alcohols, the kinetic resolution proceeds efficiently and optically active esters and optically active carboxylic acids can be obtained. The optical resolution of an anti-inflammatory agent, ibuprofen, has been achieved as an example of this reaction.
This is a useful method to obtain optically active esters, carboxlylic acids and alcohols efficiently from racemic alcohols and achiral carboxylic acids, or racemic carboxylic acids and achiral alcohols under mild conditions. It could be applied to the synthesis of various optically active compounds.
References
- 1)Benzotetramisole: a remarkably enantioselective acyl transfer catalyst
- 2)Kinetic resolution of racemic alcohols and racemic carboxylic acids
- a)I. Shiina, K. Nakata, Tetrahedron Lett. 2007, 48, 8314.

- b)I. Shiina, K. Nakata, Y. Onda, Eur. J. Org. Chem. 2008, 5887.

- c)I. Shiina, K. Nakata, M. Sugimoto, Y. Onda, T. Iizumi, K. Ono, Heterocycles 2009, 77, 801.

The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.