Published TCIMAIL newest issue No.198
Maximum quantity allowed is 999
Please select the quantity
Powerful Chiral Auxiliaries
No.119(March 2004)
- C1325
- (-)-10,2-Camphorsultam (1a)
- C1324
- (+)-10,2-Camphorsultam (1b)
- C1682
- N-(2-Carboxybenzoyl)-(-)-10,2-camphorsultam (2a)
- C1766
- N-(2-Carboxybenzoyl)-(+)-10,2-camphorsultam (2b)
- C1683
- N-(2-Carboxy-4,5-dichlorobenzoyl)-(-)-10,2-camphorsultam (3a)
- C1767
- N-(2-Carboxy-4,5-dichlorobenzoyl)-(+)-10,2-camphorsultam (3b)

Camphorsultams, 1a and 1b, are useful chiral auxiliaries, and their N-benzoyl derivatives can be used as activated chiral dienophiles to produce Diels-Alder adducts in high asymmetric yields. In connection with the study of asymmetric induction potency, Harada et al. have developed a method to determine the optical resolution and the absolute configuration of chiral carboxylic acids using 1.1) In addition, they have examined a method to introduce linkers connecting 1 with alcohol moiety to apply this method further to the alcohols, and it was found that phthalic acid was an excellent linker.
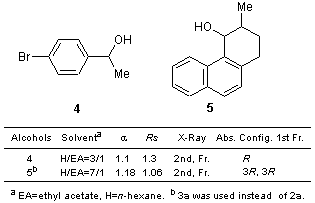
For example, the diastereomers prepared by the reaction of 2a with alcohol 4 were separated by HPLC on silica gel, and the single crystal suitable for X-ray analysis has been obtained from the second fraction, thus, making it possible to determine that the alcohol moiety of the second fraction has (S)-configuration by X-ray analysis.2a) For the poorly soluble ester obtained, the elution time was long, and the single crystal suitable for X-ray analysis could not be obtained. However, this obstacle was overcome when dichlorophthalic acid 3 was used as the linker.2b) Therefore, it is possible to determine the optical resolution and the absolute configuration of various compounds by using camphorsultams 1, 2 or 3.
References
- 1)Optical resolution and X-ray crystallographic determination of the absolute stereochemistry of carboxylic acids
- 2)Optical resolution and X-ray crystallographic determination of the absolute stereochemistry of alcohols
- a)N. Harada, T. Nehira, T. Soutome, N. Hiyoshi, F. Kido, Enantiomer 1996, 1, 35.
- b)N. Harada, N. Koumura, M. Robillard, Enantiomer 1997, 2, 303.
- c)M. Kosaka, T. Sugito, Y. Kasai, S. Kuwahara, M. Watanabe, N. Harada, G. E. Job, A. Shvet, W. H. Pirkle, Chirality 2003, 15, 324.

The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
