Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
Please select the quantity
Organocatalysts for Asymmetric Michael Additional Reactions
The two prolinol derivatives 1 and 2, known as Hayashi-Jørgensen catalysts are utilized in various types of asymmetric reactions. For instance, Hayashi et al. have reported an asymmetric Michael addition of nitroalkenes and aldehydes, which gives syn adducts with high enantio- and diastereoselectivities.1) Furthermore, utilizing this reaction as a key early-stage step allowed them to achieve a short synthesis of the anti-flu drug oseltamivir phosphate.2) Additionally, Jørgensen’s group have reported a tandem Michael/intramolecular Morita-Baylis-Hillman reaction to afford cyclohexanone derivatives.3) Both 1 and 2 have been further used as an essential tool in the asymmetric total synthesis of natural products.4)
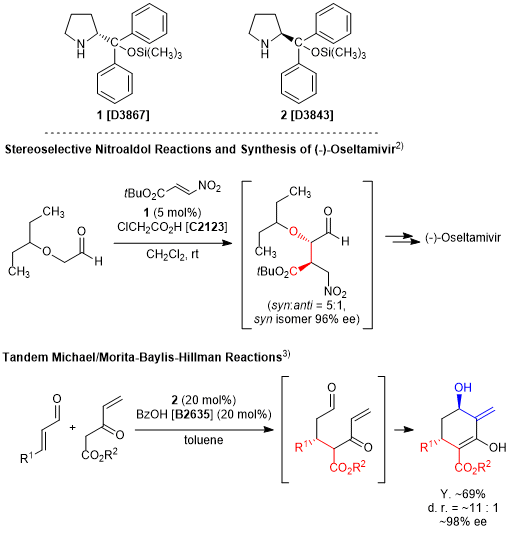
Related Products
- D3867
- (R)-(+)-α,α-Diphenyl-2-pyrrolidinemethanol Trimethylsilyl Ether
- D3843
- (S)-(-)-α,α-Diphenyl-2-pyrrolidinemethanol Trimethylsilyl Ether
References
- 1)Diphenylprolinol Silyl Ethers as Efficient Organocatalysts for the Asymmetric Michael Reaction of Aldehydes and Nitroalkenes
- 2)High-Yielding Synthesis of the Anti-Influenza Neuramidase Inhibitor (-)-Oseltamivir by Three “One-Pot” Operations
- 3)An Unexpected Organocatalytic Asymmetric Tandem Michael/Morita–Baylis–Hillman Reaction
- 4)Enantioselective Organocatalytic Michael/Aldol Sequence: Anticancer Natural Product (+)-trans-Dihydrolycoricidine
