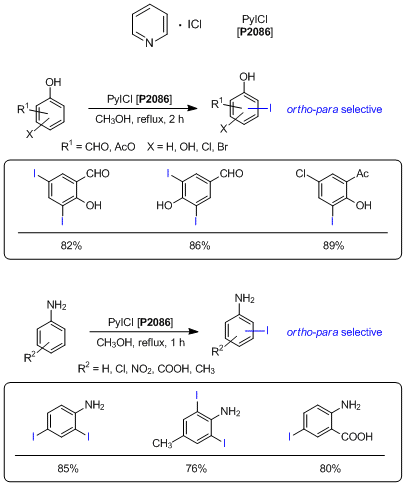
Pyridine iodine monochloride (PyICl) is a pale yellow solid
and can be used as an electrophilic iodinating reagent. While iodine monochloride exists in the solid state under
ambient temperature with a low melting point, PyICl is a solid compound having a melting point over 100 °C. Its
remarkable property enables it to be handled easily. Furthermore, general electrophilic iodinating reagents such as
the
Barluenga reagent (IPy
2BF
4) require excess amount of
some acids to accomplish iodinations. In a case using PyICl as an iodinating reagent, acids are not required to
iodinate and formyl or acetyl group substituted phenols and anilines are iodinated with
ortho-
para
selectivity under near-neutral conditions. As described above, PyICl is expected to be used for a mild and efficient
electrophilic iodinating reagent.