Kuninobu, Kanai
et al. have reported an iridium-catalyzed regioselective silylation of aryl compounds.
1) According to their results, in the presence of
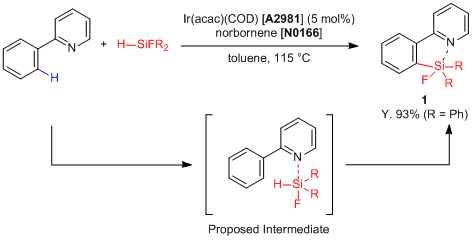
Ir(acac)(COD) catalyst, benzene derivatives possessing the directing group like a 2-pyridyl group successfully react with fluorinated hydrosilanes to proceed with the
ortho-selective C-H silylation, and the desired silafluorene equivalents
1 are given. This reaction indicates that Lewis acidity of a silicon atom is important to promote the silylation because the reaction is not promoted when using a non-fluorinated hydrosilane, and this silylation proceeds with an interaction between fluorinated hydrosilanes and the Lewis basic 2-pyridyl group. Silafluorene equivalents show strong fluorescence and high quantum yield,
2) so that this reaction is expected to be applied for development of organic materials.