Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Please select the quantity
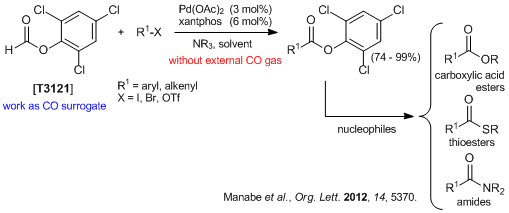
A Practical Crystalline Carbon Monoxide Surrogate
Manabe et al. have reported that 2,4,6-Trichlorophenyl formate is a highly reactive and easily accessible crystalline carbon monoxide (CO) surrogate in synthetic organic chemistry.1-3) 2,4,6-Trichlorophenyl formate undergoes facile decarbonylation in the presence of trialkylamine to afford CO and 2,4,6-trichlorophenol. The in situ generated CO and 2,4,6-trichlorophenol are successfully applied to the Pd-catalyzed carbonylation of aryl/alkenyl halides and triflates to give their 2,4,6-trichlorophenol ester. The obtained trichlorophenyl esters can be readily converted to a variety of carboxylic acid derivatives by using various nucleophiles.