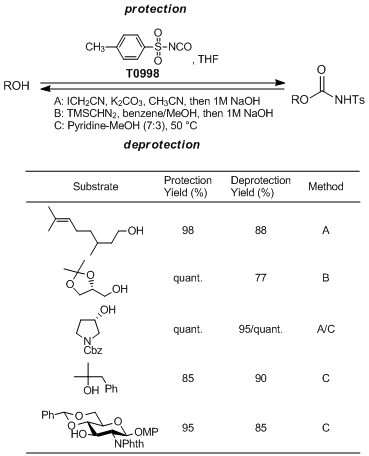
Manabe
et al. have developed the sulfonylcarbamate group as a novel hydroxy protecting group and reported its utility. According to their results, the sulfonylcarbamate group can easily be introduced by the reaction of alcohols with
p-toluenesulfonyl isocyanate under neutral conditions. This protecting group has the unique property that it is completely stable under strongly basic conditions, while it can be deprotected under extremely mild basic conditions such as in pyridine-MeOH. Furthermore, the deprotection can be achieved under strongly basic conditions after alkylation at the nitrogen atom of the carbamate group. Since this protection-deprotection reaction is carried out under mild conditions with operational simplicity, it can be applied to a wide range of substrates.