Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 1291-32-3 | Product Number: Z0007
Zirconocene Dichloride

Purity: >97.0%(T)
Synonyms:
- Bis(cyclopentadienyl)zirconium Dichloride
Product Documents:
| Size | Unit Price | Belgium | Japan* |
|---|---|---|---|
| 5G |
€36.00
|
2 | ≥80 |
| 25G |
€115.00
|
6 | ≥40 |
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | Z0007 |
| Purity / Analysis Method | >97.0%(T) |
| Molecular Formula / Molecular Weight | C__1__0H__1__0Cl__2Zr = 292.31 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Moisture Sensitive |
| CAS RN | 1291-32-3 |
| PubChem Substance ID | 87577925 |
| SDBS (AIST Spectral DB) | 5090 |
| MDL Number | MFCD00003726 |
Specifications
| Appearance | White to Almost white powder to crystal |
| Purity(Chelometric Titration) | min. 97.0 % |
| Purity(Argentometric Titration) | min. 97.0 % |
Properties (reference)
| Melting Point | 244 °C |
| Solubility (soluble in) | Chloroform, Benzene |
GHS
| Pictogram |


|
| Signal Word | Danger |
| Hazard Statements | H302 + H312 + H332 : Harmful if swallowed, in contact with skin or if inhaled. H314 : Causes severe skin burns and eye damage. H290 : May be corrosive to metals. |
| Precautionary Statements | P260 : Do not breathe dust. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection/ hearing protection. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. |
Related Laws:
| EC Number | 215-066-8 |
| RTECS# | ZH7525000 |
Transport Information:
| UN Number | UN3261 |
| Class | 8 |
| Packing Group | III |
| HS Number | 2931900090 |
Application
Zirconocene-Catalyzed Transamidation of Primary Amides with Amines
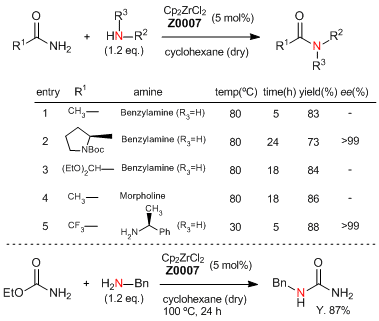
Typical procedure (entry 1):
A dried tube is charged with zirconocene dichloride (58.5 mg, 5 mol%), amide species (if it is solid), amine species (if it is solid), and the tube is sealed and purged with nitrogen gas. After which a solution of anhydrous cyclohexane or heptane (4 mL) containing acetamide (236 mg, 4 mmol) and benzylamine (424 micro-L, 4.8 mmol) is added to the reaction tube, and the tube is heated at 80 °C for 5 h. After being allowed to cool to room temperature, the reaction mixture is quenched using methanol (5 mL) and the solvent is removed under reduced pressure. Dichloromethane (50 mL) is added and the organic layer washed with water (20 mL). The organic layer is separated and the aqueous layer is extracted with dichloromethane (2 x 50 mL), the organics are combined and dried over MgSO4. The reaction mixture is filtered and concentrated under reduced pressure. The residue is purified by column chromatography (eluent: CH2Cl2 / MeOH = 10 / 1) to give N- benzylacetamide as an off-white solid (497 mg, 83% yield).
A dried tube is charged with zirconocene dichloride (58.5 mg, 5 mol%), amide species (if it is solid), amine species (if it is solid), and the tube is sealed and purged with nitrogen gas. After which a solution of anhydrous cyclohexane or heptane (4 mL) containing acetamide (236 mg, 4 mmol) and benzylamine (424 micro-L, 4.8 mmol) is added to the reaction tube, and the tube is heated at 80 °C for 5 h. After being allowed to cool to room temperature, the reaction mixture is quenched using methanol (5 mL) and the solvent is removed under reduced pressure. Dichloromethane (50 mL) is added and the organic layer washed with water (20 mL). The organic layer is separated and the aqueous layer is extracted with dichloromethane (2 x 50 mL), the organics are combined and dried over MgSO4. The reaction mixture is filtered and concentrated under reduced pressure. The residue is purified by column chromatography (eluent: CH2Cl2 / MeOH = 10 / 1) to give N- benzylacetamide as an off-white solid (497 mg, 83% yield).
References
Application
for Stereoselective Glycosidation

References
- for O-glycosidation
- for C-glycosidation
PubMed Literature
Articles/Brochures
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.




