Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 132-16-1 | Product Number: P0774
Iron(II) Phthalocyanine

Purity: >97.0%(T)
Synonyms:
- Phthalocyanine Iron(II)
Product Documents:
| Size | Unit Price | Belgium | Japan* |
|---|---|---|---|
| 5G |
€26.00
|
3 | ≥80 |
| 25G |
€87.00
|
10 | ≥100 |
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | P0774 |
| Purity / Analysis Method | >97.0%(T) |
| Molecular Formula / Molecular Weight | C__3__2H__1__6FeN__8 = 568.38 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 132-16-1 |
| Reaxys Registry Number | 15506728 |
| PubChem Substance ID | 87574915 |
| SDBS (AIST Spectral DB) | 5223 |
| MDL Number | MFCD00015953 |
Specifications
| Appearance | Dark purple powder to crystal |
| Purity(Chelometric Titration) | min. 97.0 % |
Properties (reference)
GHS
Related Laws:
| EC Number | 205-047-2 |
Transport Information:
| HS Number | 3204170090 |
Application
Catalytic Mitsunobu Reaction with an Iron Catalyst and Atmospheric Oxygen
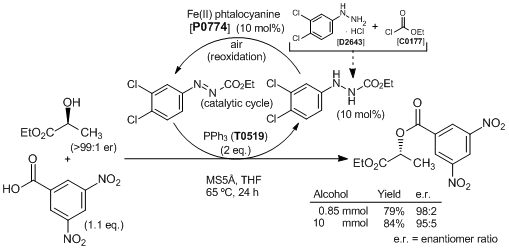
Typical procedure: A mixture of ethyl L-(-)-lactate (100 mg, 0.85 mmol), 3,5-dinitrobenzoic acid (198 mg, 0.935 mmol), triphenylphosphine (446 mg, 1.7 mmol), ethyl 2-(3,4-dichlorophenyl)hydrazinecarboxylate (21.2 mg, 0.085 mmol), iron(II) phthalocyanine (48.3 mg, 0.085 mmol) and activated MS5Å (400 mg) in THF (1.7 mL) is heated at 65 °C for 24 h under air (balloon). After the reaction mixture is cooled to room temperature and filtered, the solvent is removed under reduced pressure. The residue is purified by silica gel chromatography (eluent: n-hexane/EtOAc = 6:1). Ethyl 2-(3,4-dichlorophenyl)azocarboxylate (20.1 mg, 0.0813 mmol, 96%) is recovered from the first fraction as a red solid. The second fraction gives (R)-1-ethoxycarbonylethyl 4-nitrobenzoate (209 mg, 0.668 mmol, 79%, 98:2 e.r.) as a white solid.
References
Application
Selective Nitro Reduction with an Iron-based Catalyst
.gif)
Typical Procedure: Hydrazine hydrate (2 eq.) is added to a mixture of nitro compound (0.67 mmol) and catalyst (0.5 mol%) in water/ethanol (1:1) or ethylene glycol (10 mL). The reaction solution is refluxed to reflux at 120°C and the progress of the reaction is monitored by TLC (silica gel; hexane―ethyl acetate) and GC-MS. Upon completion, the reaction mixture is cooled to ambient temperature and extracted with ethyl acetate (3 × 5 mL). The combined organic fractions are (washed with water in cases where ethylene glycol is used as solvent) dried under reduced pressure. The product mixture is subjected to column chromatography (silica 230-400; hexane―ethyl acetate).
References
PubMed Literature
Articles/Brochures
TCIMAIL
[Research Articles] Catalytic Mitsunobu Reaction with an Iron Catalyst and Atmospheric Oxygen[Research Articles] Selective Nitro Reduction with an Iron-Based Catalyst
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.





