Published TCIMAIL newest issue No.200
Maximum quantity allowed is 999
| Size | Unit Price | Belgium | Japan* |
Shipping Information

|
|---|---|---|---|---|
| 10MG |
€143.00
|
1 | Contact Us |
* Stock available in Belgium: Shipment on the same day
* Stock available in Japan: Please check the Shipping Simulation for estimated shipments. (excludes regulated items and dry ice shipments)
| Product Number | B4195 |
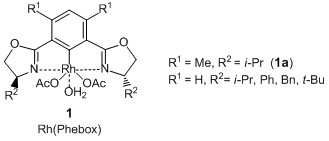
| Molecular Formula / Molecular Weight | C__2__4H__3__5N__2O__7Rh = 566.46 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 929896-28-6 |
| PubChem Substance ID | 253659893 |
| Appearance | Light yellow to Yellow to Orange powder to crystal |
| Elemental analysis(Nitrogen) | 4.40 to 5.20 % |
| NMR | confirm to structure |
| HS Number | 2843909000 |

The requested SDS is not available.
Please Contact Us for more information.
A sample C of A for this product is not available at this time.

The requested analytical chart is not available. Sorry for the inconvenience.