Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 1678540-23-2 | Product Number: E1267
Ethyl (11bR)-4-Amino-2,6-bis(3,5-di-tert-butylphenyl)-4,5-dihydro-3H-cyclohepta[1,2-a:7,6-a']dinaphthalene-4-carboxylate
![Ethyl (11bR)-4-Amino-2,6-bis(3,5-di-tert-butylphenyl)-4,5-dihydro-3H-cyclohepta[1,2-a:7,6-a']dinaphthalene-4-carboxylate No-Image](/medias/E1267.jpg?context=bWFzdGVyfHJvb3R8NzE4MDJ8aW1hZ2UvanBlZ3xhREV6TDJnMVlTODRPVEl4TmpZek5qWTRNalUwTDBVeE1qWTNMbXB3Wnd8YTE2MGM4Y2FhYzFlMDNlYjEwYTk5NTg1NDYwOTc5ZmE1M2EwNDhiYTFiZjM5ZDlkY2VjYjE4ODM3MmM1OWJjYQ)
Purity: >97.0%(HPLC)
Synonyms:
- (11bR)-4-Amino-2,6-bis(3,5-di-tert-butylphenyl)-4,5-dihydro-3H-cyclohepta[1,2-a:7,6-a']dinaphthalene-4-carboxylic Acid Ethyl Ester
Product Documents:
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 50MG |
€266.00
|
1 | 2 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | E1267 |
| Purity / Analysis Method | >97.0%(HPLC) |
| Molecular Formula / Molecular Weight | C__5__4H__6__3NO__2 = 758.10 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Refrigerated (0-10°C) |
| Condition to Avoid | Heat Sensitive |
| CAS RN | 1678540-23-2 |
| PubChem Substance ID | 354334205 |
Specifications
| Appearance | White to Almost white powder to crystal |
| Purity(HPLC) | min. 97.0 area% |
Properties (reference)
GHS
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
Related Laws:
Transport Information:
| HS Number | 2922498590 |
Application
Decarboxylative Asymmetric Chlorination Using an Asymmetric Catalyst

Experimental procedure:
To a stirred solution of 1 (10 mol%), N-chlorosuccinimide (1.5 eq.) in toluene is added α-alkyl-β-ketocarboxylic acid (1 eq.) and the reaction mixture is stirred under darkness. After completion, the resulting mixture is subjected directly silica gel column chromatography (hexane:dichloromethane) to give the corresponding α-chloroketone.
To a stirred solution of 1 (10 mol%), N-chlorosuccinimide (1.5 eq.) in toluene is added α-alkyl-β-ketocarboxylic acid (1 eq.) and the reaction mixture is stirred under darkness. After completion, the resulting mixture is subjected directly silica gel column chromatography (hexane:dichloromethane) to give the corresponding α-chloroketone.
References
- Enantioselective decarboxylative chlorination of β-ketocarboxylic acids
Application
An Enantioselective Fluorination of α-Branched Aldehydes Using Newly Developed Chiral Amine Catalyst
div class="blockC">
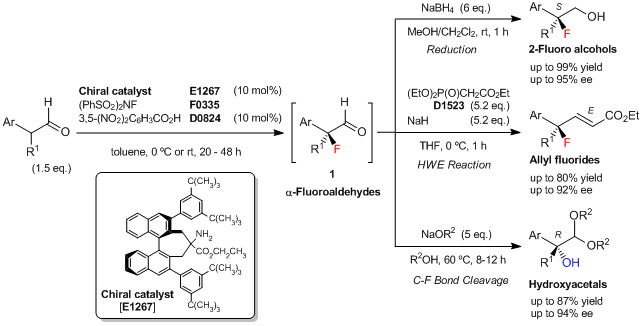
Typical Procedure (synthesis of 2-fluoro alcohol; Ar=Ph, R1=CH3):
To a solution of the chiral amine catalyst [E1267] (20 mg, 0.026 mmol, 10 mol%) in toluene (0.54 mL) is added 3,5-dinitrobenzoic acid (5.5 mg, 0.026 mmol, 10 mol%), 2-phenylpropionaldehyde (52 mg, 0.39 mmol, 1.5 eq.), and N-fluorobenzenesulfonimide (NFSI) (0.26 mmol, 82 mg, 1 eq.) at 0 °C. The reaction mixture is stirred at 0 °C for 48 h, then poured into CH3OH/CH2Cl2 (1:4, 1.3 mL) at 0 °C. To this solution, NaBH4 (1.6 mmol, 6 eq.) is added, and the mixture is stirred at room temperature for 1 h. The reaction is quenched with saturated aq.NH4Cl, and the mixture is extracted with Et2O. The organic layer is dried over Na2SO4, and concentrated. The residue is purified by silica gel chromatography (eluent: hexane/ethyl acetate = 3/1) to give (S)-2-fluoro-2-phenylpropan-1-ol (34.5 mg, 86% yield based on NFSI, 95% ee) as a white solid.
To a solution of the chiral amine catalyst [E1267] (20 mg, 0.026 mmol, 10 mol%) in toluene (0.54 mL) is added 3,5-dinitrobenzoic acid (5.5 mg, 0.026 mmol, 10 mol%), 2-phenylpropionaldehyde (52 mg, 0.39 mmol, 1.5 eq.), and N-fluorobenzenesulfonimide (NFSI) (0.26 mmol, 82 mg, 1 eq.) at 0 °C. The reaction mixture is stirred at 0 °C for 48 h, then poured into CH3OH/CH2Cl2 (1:4, 1.3 mL) at 0 °C. To this solution, NaBH4 (1.6 mmol, 6 eq.) is added, and the mixture is stirred at room temperature for 1 h. The reaction is quenched with saturated aq.NH4Cl, and the mixture is extracted with Et2O. The organic layer is dried over Na2SO4, and concentrated. The residue is purified by silica gel chromatography (eluent: hexane/ethyl acetate = 3/1) to give (S)-2-fluoro-2-phenylpropan-1-ol (34.5 mg, 86% yield based on NFSI, 95% ee) as a white solid.
References
PubMed Literature
Articles/Brochures
TCIMAIL
[Research Articles] Decarboxylative Asymmetric Chlorination Using an Asymmetric Catalyst[Research Articles] An Enantioselective Fluorination of α-Branched Aldehydes Using Newly Developed Chiral Amine Catalyst
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.



![Ethyl (11bR)-4-Amino-2,6-bis(3,5-di-tert-butylphenyl)-4,5-dihydro-3H-cyclohepta[1,2-a:7,6-a']dinaphthalene-4-carboxylate Ethyl (11bR)-4-Amino-2,6-bis(3,5-di-tert-butylphenyl)-4,5-dihydro-3H-cyclohepta[1,2-a:7,6-a']dinaphthalene-4-carboxylate](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)

