Maintenance Notice (3:15 AM - 2:55 PM May 24, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Materials Science News May 2025 | [Product Highlights] Endogenous Biotin-Blocking Reagent... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
Redox Potentials of Visible Light Photoredox Catalysts
Metal Complex Calalysts
| Chemical Structure | Product Name | Product No. | Redox Potentials / V vs. SCE in CH3CN | Reference | ||
|---|---|---|---|---|---|---|
 | Ir(dF(CF3)ppy)2(d(CF3)bpy)]PF6 | B6451 | PC*/PC− | +1.68 | 1) | |
| PC+/PC | +1.94 | |||||
| PC/PC− | −0.69 | |||||
| PC+/PC* | −0.43 | |||||
 | Ir[dF(CF3)ppy]2(bpy)PF6 | B6161 | PC*/PC− | +1.32 | 2) | |
| PC+/PC | +1.69 | |||||
| PC/PC− | −1.37 | |||||
| PC+/PC* | −1.00 | |||||
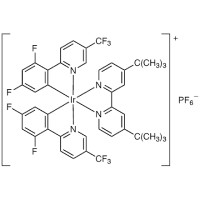 | [Ir[dF(CF3)ppy]2(dtbbpy)]PF6 | D5817 | PC*/PC− | +1.21 | 2) | |
| PC+/PC | +1.69 | |||||
| PC/PC− | −1.37 | |||||
| PC+/PC* | −0.89 | |||||
 | [Ir(dF(Me)ppy)2(dtbbpy)]PF6 | B6254 | PC*/PC− | +0.99 | 3) | |
| PC/PC− | −1.41 | |||||
 | [Ir(ppy)2(dtbbpy)]PF6 | D4887 | PC*/PC− | +0.66 | 2) | |
| PC+/PC | +1.21 | |||||
| PC/PC− | −1.51 | |||||
| PC+/PC* | −0.96 | |||||
 | Ir(ppy)3 Ir(ppy)3 (purified by sublimation) | T3716 T1946 | PC*/PC− | +0.31 | 4) | |
| PC+/PC | +0.77 | |||||
| PC/PC− | −2.19 | |||||
| PC+/PC* | −1.73 | |||||
 | Ru(bpy)3(PF6)2 | T3435 | PC*/PC− | +0.77 | 4) | |
| PC+/PC | +1.29 | |||||
 | Ru(bpy)3Cl2 Hexahydrate | T1655 | PC/PC− | −1.33 | ||
| PC+/PC* | −0.81 | |||||
 | [Ru(phen)3](PF6)2 | T3208 | PC*/PC− | +0.82 | 4) | |
| PC+/PC | +1.26 | |||||
| PC/PC− | −1.36 | |||||
| PC+/PC* | −0.87 | |||||
Organic Catalysts
| Chemical Structure | Product Name | Product No. | Redox Potentials / V vs. SCE in CH3CN | Reference | ||
|---|---|---|---|---|---|---|
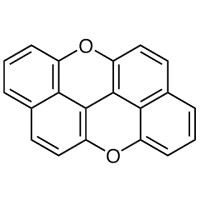 | peri-Xanthenoxanthene | X0083 | PC•+/PC | +0.82 | 5) | |
| PC•+/PC* | −1.76 | |||||
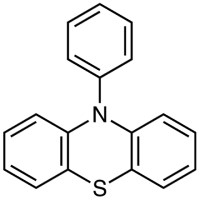 | 10-Phenylphenothiazine (= PTH) | P2470 | PC•+/PC− | +0.68 | 6) | |
| PC•+/PC* | −2.1 −1.7(T) | |||||
 | 9-Mesityl-10-methylacridinium Perchlorate | M1774 | PC+*/PC• | +2.06 | 7) | |
| PC+/PC• | −0.57 | |||||
 | Eosin Y | T0035 | PC*/PC•− | +0.83 | 6) | |
| PC•+/PC | +0.78 | |||||
| PC/PC•− | −1.06 | |||||
| PC•+/PC* | −1.11 | |||||
 | 2,4,6-Triphenylpyrylium Tetrafluoroborate | T3968 | PC+*/PC• | +2.3 | 7) | |
| PC+/PC• | −0.35 | |||||
References
- 1) Catalytic Carbocation Generation Enabled by the Mesolytic Cleavage of Alkoxyamine Radical Cations
- 2) Principles and Applications of Photoredox Catalysis:Trifluoromethylation and Beyond
- 3) Photocatalytic Generation of Aminium Radical Cations for C–N Bond Formation
- 4) Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis
- 5) Metal-free atom transfer radical polymerization with ppm catalyst loading under sunlight
- 6) The advent and development of organophotoredox catalysis
- 7) MacMillan lab's photocatalysts chart