Published TCIMAIL newest issue No.198
Maximum quantity allowed is 999
Please select the quantity
A Catalytic Strategy for Regioselective Arylethylamine Synthesis
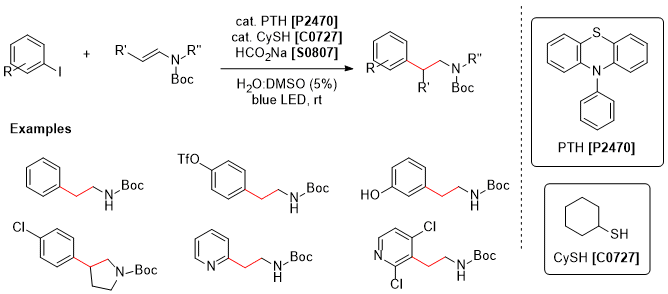
Jui and co-workers have recently reported a new method to install the pharmacologically important ethylamine moiety to a variety of aryl substrates in good yield and substrate scope. The method takes advantage of a SET-HET dual catalytic cycle utilizing 10-phenylprenothiazine (PTH) and cyclohexanethiol (CySH) respectively. Aryl iodides in the presence of these catalysts readily generates a true aryl radical which then combines with protected vinyl amines via a radical process. The reaction conditions work cleanly on both electron rich and poor aryl groups, and vinyl amines are tolerant to beta branching. Due to the frequency in which arylethylamines appear in biologically active molecules, this metal-free method is anticipated to see significant usage in medicinal chemistry, drug development, and synthesis.
Related Products
References
- Catalytic Strategy for Regioselective Arylethylamine Synthesis

