Published TCIMAIL newest issue No.198
Maximum quantity allowed is 999
Please select the quantity
A Guanidinylating Reagent without Using Mercury Salts
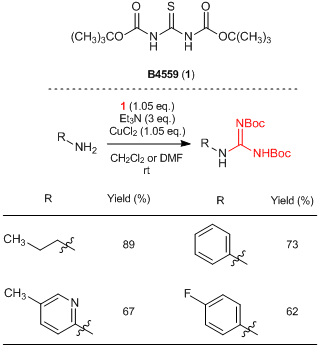
1,3-Bis(tert-butoxycarbonyl)thiourea(1) is a thiourea derivative and it can be used for the guanydinylation of amines. Generally, mercury salts are needed to effect a nucleophilic addition of amines to a thiocarbonyl moiety in thioureas. On the other hand, 1 can convert an amino group of primary amines into a guanidino group without using mercury salts, by the treatment of copper(II) chloride in the presence of triethylamine. This reaction proceeds under mild reaction conditions and various amines such as alkylamines and less nucleophilic aniline derivatives are susceptible to this transformation. It is known that some compounds such as tetrodotoxin and zanamivir have shown strong bioactivities. Thus, 1 is expected to be used as a strong tool for medicinal research because guanidinylations can be performed without using harmful mercury salts.
References
- Copper(II) chloride promoted transformation of amines into guanidines and asymmetrical N,N’-disubstituted guanidines

