Published TCIMAIL newest issue No.198
Maximum quantity allowed is 999
Please select the quantity
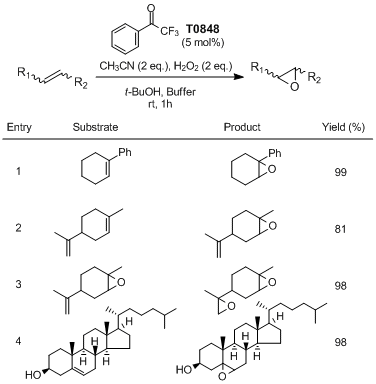
An Epoxidation of Alkenes using H2O2 (aq.) and 2,2,2-Trifluoroacetophenone
Limnios and Kokotos have reported the environmentally friendly epoxidation of alkenes using 2,2,2-trifluoroacetophenone as an organocatalyst in combination with a green oxidant aqueous H2O2. Various olefins are epoxidized chemoselectively in high to quantitative yields utilizing 2-5 mol% of the organocatalyst loading in acetonitrile and tert-butyl alcohol.