Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
Please select the quantity
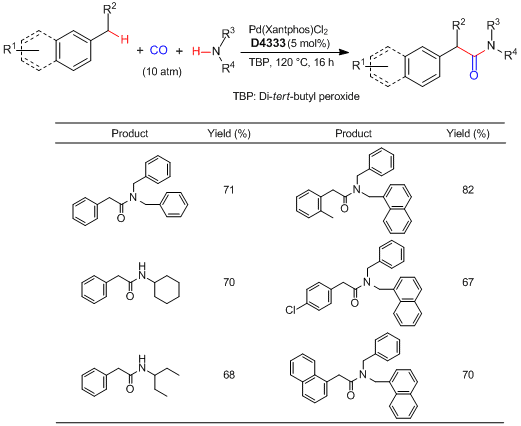
Synthesis of Arylacetamides via Benzylic C-H Bond Activation using a Palladium Catalyst
Huang et al. have reported the palladium-catalyzed oxidative aminocarbonylation via benzylic C-H bond activation. According to their results, under the presence of CO and di-tert-butyl peroxide (=TBP), benzylic C-H bonds of alkyl aromatics are selectively activated by the action of Pd(Xantphos)Cl2 catalyst. Subsequent aminocarbonylation with amines successfully proceeds to afford the corresponding arylacetamides. This reaction can be applied to substrates bearing a halogen substituent, and a subsequent cross-coupling reaction facilitates expedient synthesis of complex arylacetamides. By using this protocol, the marketed drug ibuprofen can be easily obtained.