Maintenance Notice (5:30 AM - 12:00 PM December 21, 2024): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 1G |
£45.00
|
5 | ≥100 |
|
| 5G |
£145.00
|
2 | ≥100 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | T2584 |
| Purity / Analysis Method | >98.0%(T) |
| Molecular Formula / Molecular Weight | C__1__2H__2__8BF__4P = 290.13 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Packaging and Container | 1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 131274-22-1 |
| Reaxys Registry Number | 8813613 |
| PubChem Substance ID | 125307337 |
| MDL Number | MFCD03426990 |
| Appearance | White to Almost white powder to crystal |
| Purity(Iodometric Titration) | min. 98.0 % |
| NMR | confirm to structure |
| Melting Point | 261 °C |
| Pictogram |


|
| Signal Word | Danger |
| Hazard Statements | H302 : Harmful if swallowed. H314 : Causes severe skin burns and eye damage. |
| Precautionary Statements | P260 : Do not breathe dust. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection/ hearing protection. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. |
| UN Number | UN1759 |
| Class | 8 |
| Packing Group | III |
| HS Number | 2931599090 |
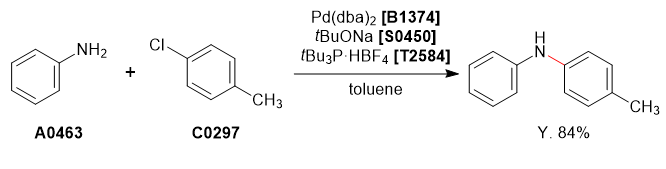
Aniline (1.0 g, 10.7 mmol) and 4-chlorotoluene (1.4 g, 11.8 mmol) were dissolved in degassed toluene (10 mL) under nitrogen atmosphere. To this solution was added sodium tert-butoxide (1.54 g, 16.1 mmol), tri-tert-butylphosphonium tetrafluoroborate (31.1 mg, 0.1 mmol) and Pd(dba)2 (61.7 mg, 0.1 mmol, 1.5mol%). The reaction mixture was refluxed for 24 h and cooled to room temperature and quenched with H2O (10 mL). The organic phase was separated and washed with brine (10 mL), dried over MgSO4 (10 g), concentrated under reduced pressure to afford the crude product as a brown liquid. The crude product was purified by column chromatography on silica-gel (hexane : ethyl acetate = 95 : 5) to afford the desired product as a pale yellow crystalline solid (1.21 g, 6.6 mmol, 62%).
Toluene was degassed by bubbling with N2 gas for 30 min.
The reaction mixture was analyzed by TLC (hexane : ethyl acetate = 1 : 1, Rf = 0.30) and GC.
1H NMR (400 MHz, CDCl3); δ 7.24-7.20 (t, J = 8.7 Hz, 3H), 7.07 (d, J = 8.7 Hz, 2H), 7.00-6.88 (m, 4H), 6.88-6.84 (m, 1H), 5.58 (s, 1H), 2.29 (s, 3H).

To a 3-necked 300 mL round bottom flask was charged with diphenylamine (5.01 g, 29.6 mmol, 1.0 eq.), 4-chloroanisole (4.48 g, 31.4 mmol, 1.05 eq.) and degassed toluene (150 mL). To this solution was added Pd2(dba)3 (0.287 g, 0.131 mmol, 1 mol%), tri-tert-butylphosphonium tetrafluoroborate (0.198 g, 0.683 mmol, 2 mol%) and sodium tert-butoxide (6.34 g, 66.0 mmol, 2.2 eq.). The reaction mixture was refluxed for 16 hr under nitrogen atmosphere. After cooled to room temperature, the reaction was diluted with CH2Cl2 (300 mL). The suspension was filtered and the filtrate was dried over Na2SO4 and concentrated under reduced pressure to afford the crude and brown solid. The crude product was purified by silica-gel column chromatography (hexane/EtOAc = 99/1 then 8/1) to afford the light brown solid (7.0 g) containing 10 mol% of diphenylamine. Removal of the residual diphenylamine by recrystallization from hexane (55 mL, 60 °C then 15 °C) gave 4-methoxytriphenylamine as a white solid (5.26 g, 65 %).
The reaction mixture was monitored by TLC (EtOAc/hexane = 1/10. Starting materials: Rf = 0.36 (diphenylamine), 0.59 (4-chloroanisole); target product: Rf = 0.46).
1H NMR (400 MHz, CD2Cl2); δ 7.26-7.17 (m, 4H), 7.10-6.98 (m, 6H), 6.98-6.91 (m, 2H), 6.89-6.82 (m, 2H), 3.79 (s, 3H).
13C NMR (101 MHz, CD2Cl2); δ 156.72, 148.56, 141.00, 129.39, 127.72, 123.12, 122.15, 115.05, 55.77.

The requested SDS is not available.
Please Contact Us for more information.
A sample C of A for this product is not available at this time.
The requested analytical chart is not available. Sorry for the inconvenience.
