Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 603-35-0 | Product Number: T0519
Triphenylphosphine

Purity: >95.0%(T)
Synonyms:
- TPP
Product Documents:
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 25G |
£16.00
|
7 | ≥100 |
|
| 100G |
£30.00
|
13 | ≥100 |
|
| 500G |
£62.00
|
24 | ≥60 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | T0519 |
| Purity / Analysis Method | >95.0%(T) |
| Molecular Formula / Molecular Weight | C__1__8H__1__5P = 262.29 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 603-35-0 |
| Reaxys Registry Number | 610776 |
| PubChem Substance ID | 87576446 |
| SDBS (AIST Spectral DB) | 2967 |
| Merck Index (14) | 9743 |
| MDL Number | MFCD00003043 |
Specifications
| Appearance | White powder to lump |
| Purity(Iodometric Titration) | min. 95.0 % |
| Melting point | 80.0 to 82.0 °C |
| Solubility in Toluene | very faint turbidity |
| NMR | confirm to structure |
Properties (reference)
| Melting Point | 81 °C |
| Boiling Point | 260 °C/25 mmHg |
| Solubility in water | Insoluble |
| Degree of solubility in water | 0.3 mg/l 25 °C |
| Solubility (soluble in) | Benzene, Toluene, Chloroform |
| Solubility (slightly sol. in) | Alcohol |
GHS
| Pictogram |


|
| Signal Word | Danger |
| Hazard Statements | H302 : Harmful if swallowed. H315 : Causes skin irritation. H319 : Causes serious eye irritation. H372 : Causes damage to organs through prolonged or repeated exposure. H373 : May cause damage to organs through prolonged or repeated exposure. H317 : May cause an allergic skin reaction. H335 : May cause respiratory irritation. H413 : May cause long lasting harmful effects to aquatic life. |
| Precautionary Statements | P273 : Avoid release to the environment. P260 : Do not breathe dust. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P314 : Get medical advice/ attention if you feel unwell. P304 + P340 + P312 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER/doctor if you feel unwell. |
Related Laws:
| EC Number | 210-036-0 |
| RTECS# | SZ3500000 |
Transport Information:
| HS Number | 2931498090 |
Application
t-Butyldimethylsilylation of Alcohols using TBS Reagents

References
- 1)A New Method for Silylation of Hydroxylic Compounds: Reaction of Phenols and Alcohols with Silanols Mediated by Diethyl Azodicarboxylate and Triphenylphosphine
- 2)Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
Application
Protection and deprotection of PMP groups
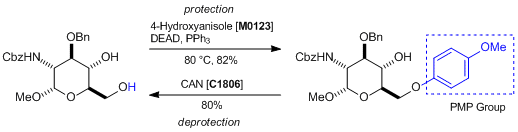
Reference
- Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
Application
Corey-Fuchs Alkyne Synthesis

Typical Procedure:
Carbon tetrabromide (34.67 g, 104.5 mmol) and anhydrous CH2Cl2 (175 mL) are added to a 500 mL round bottom flask equipped with a magnetic stir bar and septum under N2 atmosphere. The reaction flask is cooled to 0 ℃ with an ice bath for 10 min. Triphenylphosphine (54.84 g, 209.1 mmol) is added in portions over 5 min, and the dark red solution is stirred at 0 ℃ for 30 min. 3,4-Dimethoxybenzaldehyde (17.37 g, 104.5 mmol) is added and the mixture is stirred at 0 ℃ for 1 h. A 1 : 1 mixture of H2O : brine is added and the layers are separated. The aqueous layer is extracted with a 1 : 1 mixture of hexanes : CH2Cl2, and the combined organic layers are dried, filtered, and concentrated under reduced pressure. The crude material is chromatographed on SiO2 (0-15% EtOAc/hexanes) to afford the dibromide 1 (29.37 g, Y. 88%) as a yellow oil.
Then, 1 (4.41 g, 13.79 mmol, 1.0 equiv) and THF (45 mL) are added to a 250 mL round bottom flask equipped with a magnetic stir bar and septum, and the reaction flask is cooled to -78 ℃ under N2 atmosphere. 2.5M n-BuLi solution in hexane (11.6 mL) is added slowly via syringe and the reaction solution is stirred at -78 ℃ for 45 min and then at 0 ℃ for 45 min. The flask is recooled to -78 ℃, and freshly distilled methyl chloroformate (1.1 mL, 1.3 mmol, 1.0 equiv) is added slowly via syringe. The mixture is stirred at -78 ℃ for 10 min, then 0 ℃ for 1 h. Saturated aqueous NH4Cl solution is added, and the layers are separated. The aqueous layer is extracted with Et2O, and the combined organic layers are dried, filtered and concentrated under reduced pressure. The residue is chromatographed (SiO2, 2-20% EtOAc / hexanes) to afford the desired alkyne 2 (2.83 g, Y. 93%) as a white solid.
Carbon tetrabromide (34.67 g, 104.5 mmol) and anhydrous CH2Cl2 (175 mL) are added to a 500 mL round bottom flask equipped with a magnetic stir bar and septum under N2 atmosphere. The reaction flask is cooled to 0 ℃ with an ice bath for 10 min. Triphenylphosphine (54.84 g, 209.1 mmol) is added in portions over 5 min, and the dark red solution is stirred at 0 ℃ for 30 min. 3,4-Dimethoxybenzaldehyde (17.37 g, 104.5 mmol) is added and the mixture is stirred at 0 ℃ for 1 h. A 1 : 1 mixture of H2O : brine is added and the layers are separated. The aqueous layer is extracted with a 1 : 1 mixture of hexanes : CH2Cl2, and the combined organic layers are dried, filtered, and concentrated under reduced pressure. The crude material is chromatographed on SiO2 (0-15% EtOAc/hexanes) to afford the dibromide 1 (29.37 g, Y. 88%) as a yellow oil.
Then, 1 (4.41 g, 13.79 mmol, 1.0 equiv) and THF (45 mL) are added to a 250 mL round bottom flask equipped with a magnetic stir bar and septum, and the reaction flask is cooled to -78 ℃ under N2 atmosphere. 2.5M n-BuLi solution in hexane (11.6 mL) is added slowly via syringe and the reaction solution is stirred at -78 ℃ for 45 min and then at 0 ℃ for 45 min. The flask is recooled to -78 ℃, and freshly distilled methyl chloroformate (1.1 mL, 1.3 mmol, 1.0 equiv) is added slowly via syringe. The mixture is stirred at -78 ℃ for 10 min, then 0 ℃ for 1 h. Saturated aqueous NH4Cl solution is added, and the layers are separated. The aqueous layer is extracted with Et2O, and the combined organic layers are dried, filtered and concentrated under reduced pressure. The residue is chromatographed (SiO2, 2-20% EtOAc / hexanes) to afford the desired alkyne 2 (2.83 g, Y. 93%) as a white solid.
References
- Total synthesis of (+)-Lithospermic acid by asymmetric intramolecular alkylation via catalytic C-H bond activation
Application
Corey-Nicolaou Macrolactonization1)

Typical Procedure:
To a stirred solution of the hydroxy carboxylic acid 1 (125 mg, 0.422 mmol) in dry CH2Cl2 (15 mL) is added triphenylphosphine (125 mg, 0.48 mmol) followed by 2,2’-dipyridyl disulfide (105 mg, 0.48 mmol) and the mixture is stirred until TLC (ethyl acetate : hexane = 2 : 3) shows complete loss of starting material (usually 0.5 h – 2 h). The mixture is concentrated in vacuo and dissolved in dry toluene (150 mL). To the solution is added 0.006M AgBF4 solution in toluene (1.2 mL) and the mixture is heated under reflux for 12 h. The mixture is cooled and solvents removed in vacuo. The residue is purified by flash column chromatography (acetone : CH2Cl2 = 0.5 : 99.5) to afford the desired lactone 2 (95 mg, Y. 81%).
To a stirred solution of the hydroxy carboxylic acid 1 (125 mg, 0.422 mmol) in dry CH2Cl2 (15 mL) is added triphenylphosphine (125 mg, 0.48 mmol) followed by 2,2’-dipyridyl disulfide (105 mg, 0.48 mmol) and the mixture is stirred until TLC (ethyl acetate : hexane = 2 : 3) shows complete loss of starting material (usually 0.5 h – 2 h). The mixture is concentrated in vacuo and dissolved in dry toluene (150 mL). To the solution is added 0.006M AgBF4 solution in toluene (1.2 mL) and the mixture is heated under reflux for 12 h. The mixture is cooled and solvents removed in vacuo. The residue is purified by flash column chromatography (acetone : CH2Cl2 = 0.5 : 99.5) to afford the desired lactone 2 (95 mg, Y. 81%).
References
- 1)Efficient and mild lactonization method for the synthesis of macrolides
- 2)Facile synthesis of saturated eight-membered ring lactones
PubMed Literature
Articles/Brochures
TCIMAIL
[TCIMAIL No.156] Mitsunobu Reaction Using Acetone Cyanohydrin[Product Highlights] Chiral Derivatization Reagent Possessing a Pyridylthiourea Structure
[TCI Practical Example] Bromination of an Alkyl Alcohol through the Appel Reaction
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.





