Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 6553-96-4 | Product Number: T0459
2,4,6-Triisopropylbenzenesulfonyl Chloride

Purity: >97.0%(T)
Synonyms:
Product Documents:
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 25G |
£25.00
|
1 | ≥100 |
|
| 500G |
£221.00
|
1 | 7 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | T0459 |
| Purity / Analysis Method | >97.0%(T) |
| Molecular Formula / Molecular Weight | C__1__5H__2__3ClO__2S = 302.86 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Refrigerated (0-10°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Light Sensitive,Moisture Sensitive,Heat Sensitive |
| CAS RN | 6553-96-4 |
| Reaxys Registry Number | 1218575 |
| PubChem Substance ID | 87576405 |
| SDBS (AIST Spectral DB) | 10893 |
| MDL Number | MFCD00007433 |
Specifications
| Appearance | White powder to lump |
| Purity(GC) | min. 95.0 % |
| Purity(Argentometric Titration) | min. 97.0 % |
| Melting point | 94.0 to 99.0 °C |
| Solubility in Toluene | almost transparency |
| NMR | confirm to structure |
Properties (reference)
| Melting Point | 96 °C |
| Solubility (soluble in) | Toluene |
GHS
| Pictogram |

|
| Signal Word | Danger |
| Hazard Statements | H314 : Causes severe skin burns and eye damage. H290 : May be corrosive to metals. |
| Precautionary Statements | P260 : Do not breathe dust. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection/ hearing protection. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. |
Related Laws:
| EC Number | 229-479-6 |
Transport Information:
| UN Number | UN3261 |
| Class | 8 |
| Packing Group | III |
| HS Number | 2904100090 |
Application
Condensing Agent Effective for the Condensation of Hindered Amines
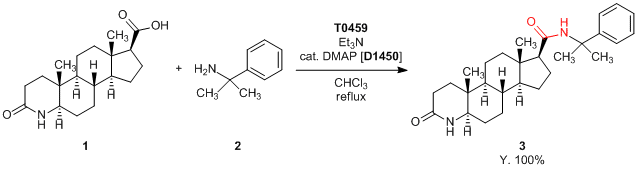
Experimental procedure: 2,4,6-Triisopropylbenzenesulfonyl chloride (144 mg, 0.48 mmol) is added in three portions every 30 min to a mixture of 1 (100 mg, 0.31 mmol), triethylamine (0.13 mL, 0.93 mmol), DMAP (5 mg) and 2 (104 mg, 0.63 mmol) in chloroform (ethanol free, 5 mL) at reflux temperature. After heating at reflux for 4 h, the reaction mixture is cooled to room temperature, poured into 1 mol/L HCl, and extracted with dichloromethane. The extracts are successively washed with dilute aq. NaHCO3, water and brine, and then dried over Na2SO4. Evaporation of the solvent affords a residue, which is purified over silica gel column chromatography (dichloromethane : acetone = 5:1 - 7:3) to yield a residue, which is crystallized from a mixture of acetone and ether to afford 147 mg (100% yield) of the product 3 as crystals.
References
- A Facile Synthesis of a Sterically Congested Amide: A Convenient Method of Steroidal 17β-Amide Synthesis from 17β-Carboxylic Acid and a Hindered Amine with 2,4,6-Triisopropylbenzenesulfonyl Chloride as a Condensing Reagent
Application
Efficient synthesis of N2-dimethylaminomethylene-2’-O-methylguanosine
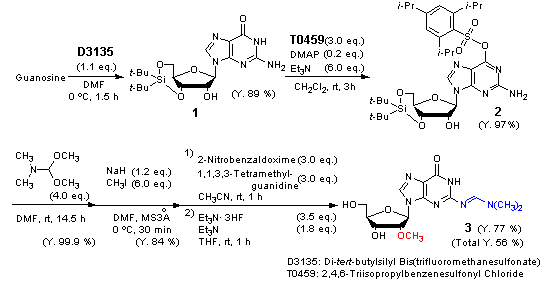
Typical procedure (protection of the O6-position of guanosine): To a suspension of 3’,5’-O-di-tert-butylsilanediylguanosine (2.12 g) in dry CH2Cl2 are added DMAP (0.122 g), Et3N (4.18 mL), and 2,4,6-triisopropylbenzenesulfonyl chloride (4.68 g) under argon. The reaction mixture is stirred at room temperature for 3 h and then the solvent is removed under reduced pressure. The residue is dissolved in ethyl acetate and the solution is washed with saturated NaHCO3 solution and brine, and dried over Na2SO4. After filtration, the solvent is evaporated in vacuo. The residue is purified by column chromatography (eluent: 50-60 % CHCl3 in hexane) to give the O6-protected guanosine as a colorless foam (3.34 g, 97%).
References
PubMed Literature
Articles/Brochures
TCIMAIL
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.





