Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
CAS RN: 540-80-7 | Product Number: N0357
tert-Butyl Nitrite
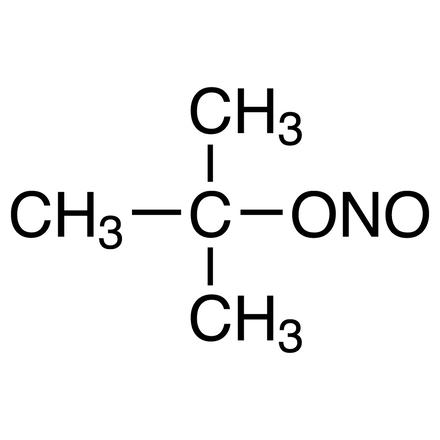
Purity: >90.0%(GC)
- Nitrous Acid tert-Butyl Ester
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 25ML |
£36.00
|
7 | ≥100 |
|
| 250ML |
£275.00
|
4 | 27 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | N0357 |
| Purity / Analysis Method | >90.0%(GC) |
| Molecular Formula / Molecular Weight | C__4H__9NO__2 = 103.12 |
| Physical State (20 deg.C) | Liquid |
| Storage Temperature | Refrigerated (0-10°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Light Sensitive,Air Sensitive,Heat Sensitive |
| CAS RN | 540-80-7 |
| Reaxys Registry Number | 1209339 |
| PubChem Substance ID | 87573709 |
| SDBS (AIST Spectral DB) | 3977 |
| Merck Index (14) | 1583 |
| MDL Number | MFCD00002055 |
| Appearance | Colorless to Yellow to Orange clear liquid |
| Purity(GC) | min. 90.0 % |
| Boiling Point | 63 °C |
| Flash point | -13 °C |
| Specific Gravity (20/20) | 0.87 |
| Refractive Index | 1.37 |
| Solubility in water | Slightly soluble, Decomposes in contact with water |
| Solubility (miscible with) | Alcohol |
| Pictogram |



|
| Signal Word | Danger |
| Hazard Statements | H302 + H332 : Harmful if swallowed or if inhaled. H370 : Causes damage to organs. H225 : Highly flammable liquid and vapour. |
| Precautionary Statements | P260 : Do not breathe mist or vapours. P210 : Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P233 : Keep container tightly closed. P264 : Wash skin thoroughly after handling. P370 + P378 : In case of fire: Use dry sand, dry chemical or alcohol-resistant foam to extinguish. P308 + P311 : IF exposed or concerned: Call a POISON CENTER/doctor. |
| EC Number | 208-757-0 |
| RTECS# | RA0802000 |
| UN Number | UN2351 |
| Class | 3 |
| Packing Group | II |
| HS Number | 2920907090 |

-
Used Chemicals
-
Procedure
-
To a solution of p-anisidine (123 mg, 1.0 mmol), p-TsOH-H2O (190 mg, 1.0 mmol, 1.0 eq.), KI (415 mg, 2.5 mmol, 2.5 eq.) in acetonitrile (5 mL) was added dropwise tBuONO (0.30 mL, 2.5 mmol, 2.5 eq.) at 0 °C and the mixture was stirred at same temperature for 30 min. Then the reaction mixture was heated 60 °C and stirred for 4 h. After quenching with water (15 mL), it was extracted with ethyl acetate (15 mL x 3) and the organic layer was washed with 2 mol/L HCl aq. (15 mL), sat. NaHCO3 aq. (15 mL), brine (15 mL), dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by column chromatography (on silica gel, ethyl acetate:hexane = 1:4) to give 4-iodeanisole as a red solid (202 mg, 86% yield).
-
Experimenter’s Comments
-
The reaction mixture was monitored by UPLC.
-
Analytical Data
-
4-iodeanisole
1H NMR (400 MHz, CDCl3); δ 7.56 (d, J = 8.0 Hz, 2H), 6.68 (d, J = 8.0 Hz, 2H), 3.78 (s, 3H).
-
Lead Reference
-
- A New, One-Step, Effective Protocol for the Iodination of Aromatic and Heterocyclic Compounds via Aprotic Diazotization of Amines

Reference
- Benzyne Click Chemistry with in Situ Generated Aromatic Azides

1. Chlorination:1)
To a mixture of copper(II)chloride (24.35 g, 181.2 mmol), dry acetonitrile (200 mL) and tert-butyl nitrite (25.7 mL, 226.5 mmol), a solution of 4-bromo-2-chloro-5-fluoroaniline (33.9 g, 151 mmol) in dry acetonitrile (200 mL) is added at 60 ℃ under nitrogen. The mixture is stirred for 30 min at 60 ℃, followed by cooling to room temperature and addition of 2M HCl (400 mL). The phases are separated, and the water phase is extracted with diethyl ether (150 mL). The combined organic phases are washed with water and dried over MgSO4. The organic phase is concentrated on the rotary evaporator and is distilled at 150 ℃/ 20 mbar, yielding colourless oil (30 g, 111 mmol, 72% yield, 90% purity).
Anhydrous copper(II) bromide (6.8 g, 30.5 mmol), tert-butyl nitrite (4.3 mL, 36 mmol), and anhydrous acetonitrile (150 mL) are added to a three-neck round-bottom flask, and the mixture is heated to 65 ℃. 2,7-Diamino-9,10-anthraquinone (2.9 g, 12 mmol) is added slowly over a period of 5 min to the reaction mixture. Nitrogen is evolving during the reaction. After nitrogen evolution have subsided, the reaction mixture is cooled to room temperature and poured into an aqueous 20% HCl solution (100 mL). The crude solid product is collected, washed with ether, and chromatographed on a silica gel column (hexane : CH2Cl2 = 1: 1) to afford the desired product (2.6 g, Y.60%) as a yellow solid.
References
- 1)Synthesis of Dioxin-like Monofluorinated PCBs: for the Use as Internal Standards for PCB Analysis.
- 2)Arylethynyl Substituted 9,10-Anthraquinones: Tunable Stokes Shifts by Substitution and Solvent Polarity.
Articles/Brochures
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts
The requested analytical chart is not available. Sorry for the inconvenience.





