Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 14024-18-1 | Product Number: I0079
Tris(2,4-pentanedionato)iron(III)

Purity: >98.0%(T)
Synonyms:
- Acetylacetone Iron(III) Salt
- Ferric(III) Acetylacetonate
- Iron(III) Acetylacetonate
Product Documents:
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 25G |
£16.00
|
8 | ≥80 |
|
| 100G |
£32.00
|
2 | 20 |
|
| 500G |
£102.00
|
1 | 8 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | I0079 |
| Purity / Analysis Method | >98.0%(T) |
| Molecular Formula / Molecular Weight | C__1__5H__2__1FeO__6 = 353.17 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 14024-18-1 |
| PubChem Substance ID | 87571482 |
| SDBS (AIST Spectral DB) | 4155 |
| MDL Number | MFCD00000020 |
Specifications
| Appearance | Red to Dark red to Brown powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
Properties (reference)
| Melting Point | 185 °C(dec.) |
| Solubility in water | Slightly soluble |
| Solubility (soluble in) | Ethanol, Acetone, Benzene, dichloromethane, Toluene, Chloroform |
GHS
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H302 : Harmful if swallowed. H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P270 : Do not eat, drink or smoke when using this product. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P301 + P312 + P330 : IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. Rinse mouth. |
Related Laws:
| EC Number | 237-853-5 |
| RTECS# | NO8960000 |
Transport Information:
| HS Number | 2914199090 |
Application
2-PySO2CF2H : Bench-stable Difluoromethylating Agent for Catalytic Difluoromethylation

Reference
- Iron-Catalyzed Di?uoromethylation of Arylzincs with Di?uoromethyl 2?Pyridyl Sulfone
Application
Iron/dppbz-catalyzed Decarboxylative Couplings of Active Esters
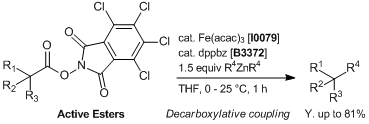
In a screwed-capped vial active ester (0.1 mmol), Fe(acac)3 (0.005 – 0.04 mmol) and dppbz (0.006 – 0.048 mmol) are weighed. The vial is then sealed, evacuated and back-filled with Ar. Then, 0.5 mL of distilled THF is added. The mixture is stirred for 5 minutes under Ar and diarylzinc reagent in THF (ca. 0.3 mol/L, 0.15 – 0.25 mmol) is added in one portion at 0 - 25 °C to the mixture and stirred at the same temperature for 1 h. After this time, the reaction is quenched with 1 mol/L HClaq, sat. NH4Claq or H2O and diluted with diethyl ether. The organic layer is separated and dried over Na2SO4 anhydrous, filtered and evaporated to dryness. Pure products are obtained after column chromatography or preparative TLC.
References
Application
Iron-Catalyzed ortho-Alkylation of Carboxamides

Typical procedure (R = benzyl): To Fe(acac)3 (17.1 mg, 0.05 mmol), dppe (29.9 mg, 0.075 mmol), and 3-methyl-N-(quinolin-8-yl)benzamide (131 mg, 0.5 mmol) are added THF (4 mL), and benzyl chloride (190 mg, 1.5 mmol). The mixture is then placed in an oil bath at 65 °C and PhMgBr (16% in THF, ca. 1 mol/L) (0.6 mL, 0.6 mmol) is added in a single portion. Upon completion of PhMgBr addition, the reaction mixture is stirred at 65 °C for 1 min. Then PhMgBr (16% in THF, ca. 1 mol/L) (1.3 mL, 1.3 mmol) is added dropwise at 65 °C for 9 min. The reaction mixture is stirred at 65 °C for 1 min and brought to rt. Subsequently the reaction mixture is quenched with a saturated solution of ammonium chloride (535 mg, 0.01 mol) and extracted with dichloromethane (3 x 3 mL). The combined organic extracts are dried over MgSO4, filtered and concentrated to give a residue. The residue is purified by silica gel flash chromatography (eluent: hexanes/EtOAc = 20:1) to give 2-benzyl-5-methyl-N-(quinolin-8-yl)benzamide (175 mg, 99% yield) as a white solid.
References
Application
Efficient Fe/Cu Co-Catalyzed C-N Cross-Coupling Reaction
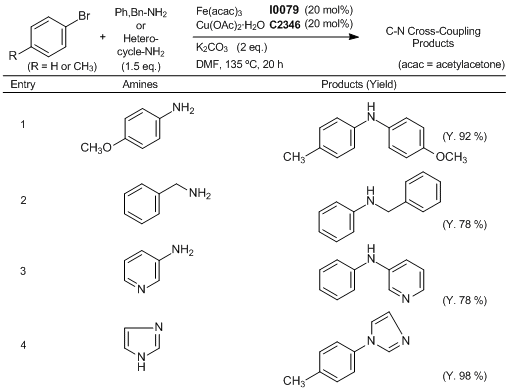
Typical procedure (Entry 1): A Schlenk tube is charged with p-anisidine (92.4 mg), K2CO3 (138 mg), Fe(acac)3 (34.4 mg), and Cu(OAc)2· H2O (20 mg). Under a nitrogen atmosphere, 4-bromotoluene (85.5 mg) is added followed by dry DMF (0.8 mL). The reaction mixture is then heated to 135 °C, and stirred for 20 h. The mixture is cooled to room temperature and diluted with diethyl ether. The resulting suspension is filtered through a celite pad and the filtrate is concentrated. The residue is purified by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate) to give 4-methoxy-N-p-tolylaniline (98.1 mg, 92 %).
References
Application
Iron-Catalyzed Cross-Coupling Reaction

Reference
PubMed Literature
Articles/Brochures
TCIMAIL
[Product Highlights] 2-PySO2CF2H : Bench-stable Difluoromethylating Agent for Catalytic Difluoromethylation[Research Articles] Fe or Ni-catalyzed Decarboxylative C-C Couplings of Active Esters
[Research Articles] Iron-Catalyzed ortho-Alkylation of Carboxamides
[Research Articles] Efficient Fe/Cu Co-Catalyzed C-N Cross-Coupling Reaction
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.





