Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 6046-93-1 | Product Number: C2346
Copper(II) Acetate Monohydrate

Purity: >95.0%(T)
Synonyms:
- Acetic Acid Copper(II) Salt Monohydrate
Product Documents:
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 25G |
£16.00
|
9 | ≥80 |
|
| 500G |
£37.00
|
7 | ≥100 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | C2346 |
| Purity / Analysis Method | >95.0%(T) |
| Molecular Formula / Molecular Weight | C__4H__6CuO__4·H__2O = 199.65 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 6046-93-1 |
| Related CAS RN | 142-71-2 |
| Reaxys Registry Number | 3730548 |
| PubChem Substance ID | 87561330 |
| MDL Number | MFCD00149570 |
Specifications
| Appearance | Blue to Dark blue powder to crystal |
| Purity(Chelometric Titration) | min. 95.0 % |
Properties (reference)
| Melting Point | 115 °C |
| Solubility in water | Soluble |
| Solubility (soluble in) | Alcohol |
| Solubility (slightly sol. in) | Ether |
GHS
| Pictogram |




|
| Signal Word | Danger |
| Hazard Statements | H302 : Harmful if swallowed. H314 : Causes severe skin burns and eye damage. H370 : Causes damage to organs. H372 : Causes damage to organs through prolonged or repeated exposure. H335 : May cause respiratory irritation. H410 : Very toxic to aquatic life with long lasting effects. |
| Precautionary Statements | P273 : Avoid release to the environment. P260 : Do not breathe dust. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection/ hearing protection. P391 : Collect spillage. P308 + P311 : IF exposed or concerned: Call a POISON CENTER/doctor. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. |
Related Laws:
| RTECS# | AG3500000 |
Transport Information:
| UN Number | UN1759 |
| Class | 8 |
| Packing Group | II |
| HS Number | 2915290000 |
Application
Synthesis of N―H Carbazoles via Ir-catalyzed Intramolecular C―H Amination
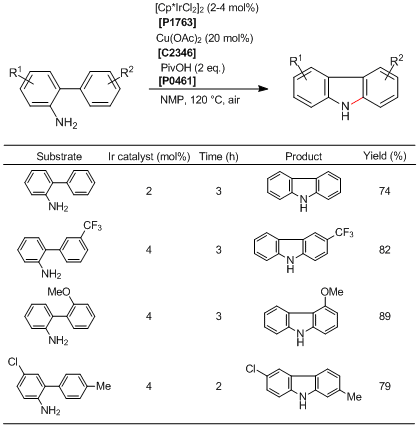
Typical Procedure:
To a 20 mL two-necked flask with a reflux condenser and a rubber cap are added 2-aminobiphenyls (0.5 mmol), [Cp*IrCl2]2 (0.01 mmol, 8.0 mg), Cu(OAc)2 (0.1 mmol, 18 mg), pivalic acid (1 mmol, 102 mg), 1-methylnaphthalene (ca. 30 mg) as an internal standard, and N-methyl-2-pyrrolidone (NMP, 3 mL). Then, the resulting mixture is stirred under air at 120 °C for 3-6 h. After cooling, the reaction mixture is extracted with ethyl acetate (100 mL). The organic layer is washed by aqueous NaHCO3 (100 mL×3), and dried over Na2SO4. After evaporation of the solvents under vacuum, the product is isolated by column chromatography on silica gel using hexane-ethyl acetate (10:1) as the eluent.
To a 20 mL two-necked flask with a reflux condenser and a rubber cap are added 2-aminobiphenyls (0.5 mmol), [Cp*IrCl2]2 (0.01 mmol, 8.0 mg), Cu(OAc)2 (0.1 mmol, 18 mg), pivalic acid (1 mmol, 102 mg), 1-methylnaphthalene (ca. 30 mg) as an internal standard, and N-methyl-2-pyrrolidone (NMP, 3 mL). Then, the resulting mixture is stirred under air at 120 °C for 3-6 h. After cooling, the reaction mixture is extracted with ethyl acetate (100 mL). The organic layer is washed by aqueous NaHCO3 (100 mL×3), and dried over Na2SO4. After evaporation of the solvents under vacuum, the product is isolated by column chromatography on silica gel using hexane-ethyl acetate (10:1) as the eluent.
References
Application
Synthesis of Carbinols

Typical procedure: Under an air atmosphere, a Schlenk tube is charged with Cu(OAc)2·H2O (4.0 mg, 0.02 mmol), dppf (16.6 mg, 0.03 mmol), arylboronic acid (0.4 mmol), aldehyde (0.2 mmol), NaOAc (49.2 mg, 0.6 mmol), and toluene (3 mL) under ice-salt (-20 °C). The mixture is stirred for 0.5 h at room temperature, refluxed for 24 h, and then cooled in a Schlenk tube to room temperature. The mixture is extracted with ethyl acetate (4×5 mL), and the organic layers are washed with water. The organic layers are dried over MgSO4, concentrated, and purified by flash column chromatography on silica gel to give the desired product.
References
PubMed Literature
Articles/Brochures
TCIMAIL
[Research Articles] Synthesis of N-H Carbazoles via Ir-catalyzed Intramolecular C-H AminationProduct Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.





