Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 108-05-4 | Product Number: A0045
Vinyl Acetate Monomer (stabilized with HQ)
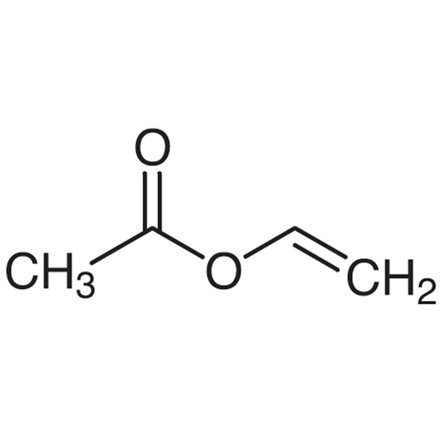
Purity: >99.0%(GC)
Synonyms:
- Acetic Acid Vinyl Ester Monomer (stabilized with HQ)
Product Documents:
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 500ML |
£16.00
|
≥40 | ≥100 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | A0045 |
| Purity / Analysis Method | >99.0%(GC) |
| Molecular Formula / Molecular Weight | C__4H__6O__2 = 86.09 |
| Physical State (20 deg.C) | Liquid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Condition to Avoid | Light Sensitive,Air Sensitive |
| CAS RN | 108-05-4 |
| Reaxys Registry Number | 1209327 |
| PubChem Substance ID | 87561748 |
| SDBS (AIST Spectral DB) | 1299 |
| Merck Index (14) | 9992 |
| MDL Number | MFCD00008713 |
Specifications
| Appearance | Colorless clear liquid |
| Purity(GC) | min. 99.0 % |
| NMR | confirm to structure |
Properties (reference)
| Melting Point | -93 °C |
| Boiling Point | 72 °C |
| Flash point | -8 °C |
| Specific Gravity (20/20) | 0.93 |
| Refractive Index | 1.40 |
| Solubility in water | Slightly soluble |
| Degree of solubility in water | 25 g/l 20 °C |
| Solubility (miscible with) | Ether, Alcohol |
| Solubility (soluble in) | Chloroform, Acetone |
GHS
| Pictogram |



|
| Signal Word | Danger |
| Hazard Statements | H332 : Harmful if inhaled. H319 : Causes serious eye irritation. H373 : May cause damage to organs through prolonged or repeated exposure. H341 : Suspected of causing genetic defects. H351 : Suspected of causing cancer. H335 : May cause respiratory irritation. H225 : Highly flammable liquid and vapour. |
| Precautionary Statements | P260 : Do not breathe dust/ fume/ gas/ mist/ vapours/ spray. P210 : Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P233 : Keep container tightly closed. P201 : Obtain special instructions before use. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P370 + P378 : In case of fire: Use dry sand, dry chemical or alcohol-resistant foam to extinguish. |
Related Laws:
| EC Number | 203-545-4 |
| RTECS# | AK0875000 |
Transport Information:
| UN Number | UN1301 |
| Class | 3 |
| Packing Group | II |
| HS Number | 2915320000 |
Application
Acetylation of Alcohols via Biotransformation using Acetylating Reagents

References
- 1)Regioselective Lipase-catalyzed Acylation of 4,6-O-benzylidene-α-and-β-D-pyranoside Derivatives Displaying a Range of Anomeric Substituents
- 2)On the Regioselective Acylation of 1,6-Anhydro-β-D-and L-Hexopyranoses Catalyzed by Lipases: Structural Basis and Synthetic Applications
- 3)Regioselective Acylation of Polyhydroxylated Natural Compounds Catalyzed by Candida Antarctica Lipase B (Novozym 435) in Organic Solvents
- 4)Protection for the Hydroxyl Group, Including 1,2-and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
Application
Acetylation of Alcohols via Transesterification using Acetylating Reagents

References
- 1)Acylation of Alcohols and Amines with Vinyl Acetates Catalyzed by Cp*2Sm(thf)2
- 2)Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
Application
Acetylation of Alcohols using Acetylating Reagents and Acid Catalysts

References
- 1)Molecular Iodine Catalyzed Selective Acetylation of Alcohols with Vinyl Acetate
- 2)Molecular Iodine in Isopropenyl Acetate (IPA): A Highly Efficient Catalyst for the Acetylation of Alcohols, Amines and Phenols under Solvent Free Conditions
- 3)Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
Application
Rhodium-Catalyzed Cross-coupling of Organoboron Compounds with Vinyl Acetate

Typical procedure: Under nitrogen atmosphere, a mixture of an arylboron compounds (0.50 mmol), [RhCl(cod)]2 (6.2 mg, 13 µmol), DPPB (11.7 mg, 28 µmol), and K3PO4 (320 mg, 1.5 mmol) is diluted with toluene (2.0 mL). Alkenyl acetate (1.1 or 2.5 mmol) and tert-amyl alcohol (0.55-1.5 mmol) are added into the resulting suspension at room temperature. The mixture is stirred at 100℃ for 24 h and then diluted with hexane (2.0 mL) or EtOAc (2.0 mL). After the filtration through a Celite pad, solvent is removed from the filtrate under reduced pressure. The residue is purified with a flash column chromatography (EtOAc-hexane) to give the desired product.
References
PubMed Literature
Articles/Brochures
TCIMAIL
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.





