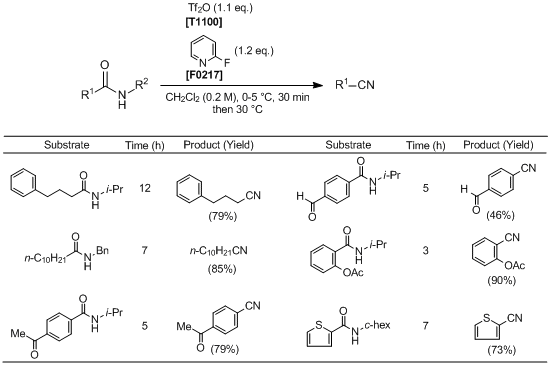
Huang
et al. have reported the transformation of aliphatic and aromatic secondary amides to nitriles. According to their results, by using
trifluoromethanesulfonic anhydride in combination with
2-fluoropyridine, secondary amides are successfully transformed into the corresponding nitriles with the elimination of an
N-alkyl group under mild reaction conditions. This reaction effectively proceeds when using amides bearing either secondary, tertiary, or benzylic
N-alkyl groups. In addition, the reaction also tolerates several labile functional groups such as esters, ketones and aldehydes. Thus this synthetic approach has attracted much attention as a useful synthetic method for nitriles.