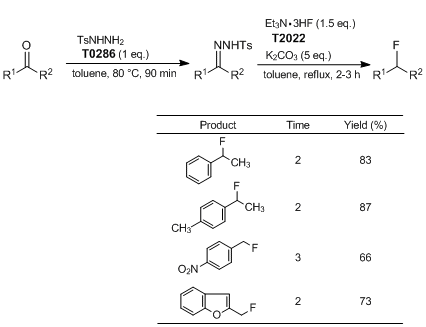
Yadav
et al. have reported deoxygenative hydrofluorination of carbonyl compounds via their tosylhydrazones. According to their results, tosylhydrazones are generated as an intermediate by the reaction of various aldehydes or ketones with
tosylhydrazide, and the subsequent reaction with
triethylamine trihydrofluoride affords the corresponding monofluoroalkanes in good to excellent yields with high purity. This reaction has attracted much attention because it can be carried out in a one-pot procedure and applied to a wide range of carbonyl compounds.