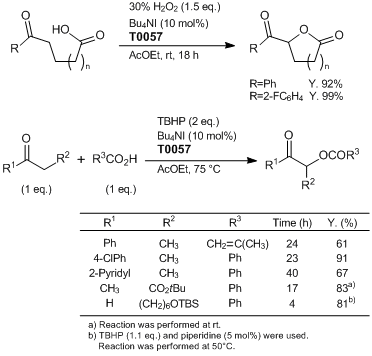
Ishihara
et al. have reported the direct α-oxyacylation of carbonyl compounds with carboxylic acids catalyzed by (hypo)iodite species. According to their results, the (hypo)iodite, which is generated in situ from a catalytic amount of
tetrabutylammonium iodide and either hydrogen peroxide or
tert-butyl hydroperoxide (TBHP) as a co-oxidant, catalyzes these α-oxyacylations. Since this reaction proceeds under mild conditions, side reactions do not proceed and the desired products can be obtained in high yields. It can be applied to a wide range of substrates, such as ketones, aldehydes and 1,3-dicarbonyl compounds.