Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Merci de sélectionner la quantité
CAS RN: 603-35-0 | Numéro de produit: T0519
Triphenylphosphine

Pureté: >95.0%(T)
Synonymes
- TPP
Documents de produit:
| Taille | Prix unitaire | Belgique | Japon * | Quantité |
|---|---|---|---|---|
| 25G |
17,00 €
|
3 | ≥100 |
|
| 100G |
34,00 €
|
7 | ≥80 |
|
| 500G |
71,00 €
|
22 | 33 |
|
*Le délai de livraison pour des produits disponibles en stock en Belgique est 1 à 2 jours
*Le délai de livraison pour des produits disponibles en stock en Japon est 1 à 2 semaines (sauf des produits réglementés et des envois avec de la glace carbonique)
| Numéro de produit | T0519 |
| Pureté / Méthode d'analyse | >95.0%(T) |
| Formule moléculaire / poids moléculaire | C__1__8H__1__5P = 262.29 |
| Etat physique (20 ° C) | Solid |
| Condition de stockage | Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 603-35-0 |
| Numéro de registre de Reaxys | 610776 |
| Identifiant de la substance PubChem | 87576446 |
| SDBS | 2967 |
| Indice Merck (14) | 9743 |
| Numéro MDL | MFCD00003043 |
Spécifications
| Appearance | White powder to lump |
| Purity(Iodometric Titration) | min. 95.0 % |
| Melting point | 80.0 to 82.0 °C |
| Solubility in Toluene | very faint turbidity |
| NMR | confirm to structure |
Propriétés
| Point de fusion | 81 °C |
| Point d'ébullition | 260 °C/25 mmHg |
| solubilité dans l'eau | Insoluble |
| Degré de solubilité dans l'eau | 0.3 mg/l 25 °C |
| Solubilité (soluble dans) | Chloroform, Benzene, Toluene |
| Solubilité (légèrement sol. In) | Alcohol |
SGH
| Pictogramme |


|
| Mot de signal | Danger |
| Mentions de danger | H302 : Nocif en cas d'ingestion. H315 : Provoque une irritation cutanée. H319 : Provoque une sévère irritation des yeux. H372 : Risque avéré d'effets graves pour les organes à la suite d'expositions répétées ou d'une exposition prolongée. H373 : Risque présumé d'effets graves pour les organes à la suite d'expositions répétées ou d'une exposition prolon H317 : Peut provoquer une allergie cutanée. H335 : Peut irriter les voies respiratoires. H413 : Peut être nocif à long terme pour les organismes aquatiques. |
| Conseils de prudence | P273 : Éviter le rejet dans l'environnement. P260 : Ne pas respirer les poussières. P264 : Se laver la peau soigneusement après manipulation. P280 : Porter des gants de protection/ un équipement de protection des yeux/ du visage. P314 : Consulter un médecin en cas de malaise. P304 + P340 + P312 : EN CAS D’INHALATION: transporter la personne à l’extérieur et la maintenir dans une position où elle peut confortablement respirer. Appeler un CENTRE ANTIPOISON/un médecin en cas de malaise. |
Lois connexes:
| Numéros CE | 210-036-0 |
| RTECS # | SZ3500000 |
Informations de transport:
| N ° SH (import / export) (TCI-E) | 2931498090 |
Application
t-Butyldimethylsilylation of Alcohols using TBS Reagents

References
- 1)A New Method for Silylation of Hydroxylic Compounds: Reaction of Phenols and Alcohols with Silanols Mediated by Diethyl Azodicarboxylate and Triphenylphosphine
- 2)Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
Application
Protection and deprotection of PMP groups
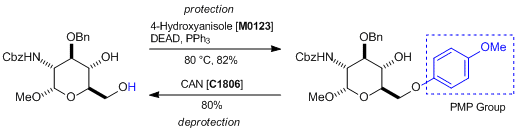
Reference
- Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
Application
Corey-Fuchs Alkyne Synthesis

Typical Procedure:
Carbon tetrabromide (34.67 g, 104.5 mmol) and anhydrous CH2Cl2 (175 mL) are added to a 500 mL round bottom flask equipped with a magnetic stir bar and septum under N2 atmosphere. The reaction flask is cooled to 0 ℃ with an ice bath for 10 min. Triphenylphosphine (54.84 g, 209.1 mmol) is added in portions over 5 min, and the dark red solution is stirred at 0 ℃ for 30 min. 3,4-Dimethoxybenzaldehyde (17.37 g, 104.5 mmol) is added and the mixture is stirred at 0 ℃ for 1 h. A 1 : 1 mixture of H2O : brine is added and the layers are separated. The aqueous layer is extracted with a 1 : 1 mixture of hexanes : CH2Cl2, and the combined organic layers are dried, filtered, and concentrated under reduced pressure. The crude material is chromatographed on SiO2 (0-15% EtOAc/hexanes) to afford the dibromide 1 (29.37 g, Y. 88%) as a yellow oil.
Then, 1 (4.41 g, 13.79 mmol, 1.0 equiv) and THF (45 mL) are added to a 250 mL round bottom flask equipped with a magnetic stir bar and septum, and the reaction flask is cooled to -78 ℃ under N2 atmosphere. 2.5M n-BuLi solution in hexane (11.6 mL) is added slowly via syringe and the reaction solution is stirred at -78 ℃ for 45 min and then at 0 ℃ for 45 min. The flask is recooled to -78 ℃, and freshly distilled methyl chloroformate (1.1 mL, 1.3 mmol, 1.0 equiv) is added slowly via syringe. The mixture is stirred at -78 ℃ for 10 min, then 0 ℃ for 1 h. Saturated aqueous NH4Cl solution is added, and the layers are separated. The aqueous layer is extracted with Et2O, and the combined organic layers are dried, filtered and concentrated under reduced pressure. The residue is chromatographed (SiO2, 2-20% EtOAc / hexanes) to afford the desired alkyne 2 (2.83 g, Y. 93%) as a white solid.
Carbon tetrabromide (34.67 g, 104.5 mmol) and anhydrous CH2Cl2 (175 mL) are added to a 500 mL round bottom flask equipped with a magnetic stir bar and septum under N2 atmosphere. The reaction flask is cooled to 0 ℃ with an ice bath for 10 min. Triphenylphosphine (54.84 g, 209.1 mmol) is added in portions over 5 min, and the dark red solution is stirred at 0 ℃ for 30 min. 3,4-Dimethoxybenzaldehyde (17.37 g, 104.5 mmol) is added and the mixture is stirred at 0 ℃ for 1 h. A 1 : 1 mixture of H2O : brine is added and the layers are separated. The aqueous layer is extracted with a 1 : 1 mixture of hexanes : CH2Cl2, and the combined organic layers are dried, filtered, and concentrated under reduced pressure. The crude material is chromatographed on SiO2 (0-15% EtOAc/hexanes) to afford the dibromide 1 (29.37 g, Y. 88%) as a yellow oil.
Then, 1 (4.41 g, 13.79 mmol, 1.0 equiv) and THF (45 mL) are added to a 250 mL round bottom flask equipped with a magnetic stir bar and septum, and the reaction flask is cooled to -78 ℃ under N2 atmosphere. 2.5M n-BuLi solution in hexane (11.6 mL) is added slowly via syringe and the reaction solution is stirred at -78 ℃ for 45 min and then at 0 ℃ for 45 min. The flask is recooled to -78 ℃, and freshly distilled methyl chloroformate (1.1 mL, 1.3 mmol, 1.0 equiv) is added slowly via syringe. The mixture is stirred at -78 ℃ for 10 min, then 0 ℃ for 1 h. Saturated aqueous NH4Cl solution is added, and the layers are separated. The aqueous layer is extracted with Et2O, and the combined organic layers are dried, filtered and concentrated under reduced pressure. The residue is chromatographed (SiO2, 2-20% EtOAc / hexanes) to afford the desired alkyne 2 (2.83 g, Y. 93%) as a white solid.
References
- Total synthesis of (+)-Lithospermic acid by asymmetric intramolecular alkylation via catalytic C-H bond activation
Application
Corey-Nicolaou Macrolactonization1)

Typical Procedure:
To a stirred solution of the hydroxy carboxylic acid 1 (125 mg, 0.422 mmol) in dry CH2Cl2 (15 mL) is added triphenylphosphine (125 mg, 0.48 mmol) followed by 2,2’-dipyridyl disulfide (105 mg, 0.48 mmol) and the mixture is stirred until TLC (ethyl acetate : hexane = 2 : 3) shows complete loss of starting material (usually 0.5 h – 2 h). The mixture is concentrated in vacuo and dissolved in dry toluene (150 mL). To the solution is added 0.006M AgBF4 solution in toluene (1.2 mL) and the mixture is heated under reflux for 12 h. The mixture is cooled and solvents removed in vacuo. The residue is purified by flash column chromatography (acetone : CH2Cl2 = 0.5 : 99.5) to afford the desired lactone 2 (95 mg, Y. 81%).
To a stirred solution of the hydroxy carboxylic acid 1 (125 mg, 0.422 mmol) in dry CH2Cl2 (15 mL) is added triphenylphosphine (125 mg, 0.48 mmol) followed by 2,2’-dipyridyl disulfide (105 mg, 0.48 mmol) and the mixture is stirred until TLC (ethyl acetate : hexane = 2 : 3) shows complete loss of starting material (usually 0.5 h – 2 h). The mixture is concentrated in vacuo and dissolved in dry toluene (150 mL). To the solution is added 0.006M AgBF4 solution in toluene (1.2 mL) and the mixture is heated under reflux for 12 h. The mixture is cooled and solvents removed in vacuo. The residue is purified by flash column chromatography (acetone : CH2Cl2 = 0.5 : 99.5) to afford the desired lactone 2 (95 mg, Y. 81%).
References
- 1)Efficient and mild lactonization method for the synthesis of macrolides
- 2)Facile synthesis of saturated eight-membered ring lactones
PubMed Litterature
Articles / Brochures
TCIMail
[TCIMAIL No.156] Mitsunobu Reaction Using Acetone Cyanohydrin[Product Highlights] Chiral Derivatization Reagent Possessing a Pyridylthiourea Structure
[TCI Practical Example] Bromination of an Alkyl Alcohol through the Appel Reaction
Documents de produit (Note : Pour certains produits, les tableaux analytiques ne sont pas disponibles.)
Fiche de sécurité (FDS)
S'il vous plaît sélectionnez la langue.
La FDS demandée n'est pas disponible.
Nous contacter pour plus d'informations.
Spécifications
CoA et autres Certificats
Veuillez remplir le numéro de lot
Le numéro de lot saisi est incorrect. Veuillez saisir uniquement 4-5 caractères alphanumériques avant le trait d'union.
Exemple de CoA
Il s'agit d'un échantillon CoA qui peut ne pas représenter un lot récemment fabriqué du produit.
Un échantillon CoA pour ce produit n'est pas disponible pour le moment.
Graphiques analytiques
Veuillez remplir le numéro de lot
Le numéro de lot saisi est incorrect. Veuillez saisir uniquement 4-5 caractères alphanumériques avant le trait d'union.
Le tableau analytique demandé n'est pas disponible. Nous sommes désolés pour ce désagrément.





