Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
CAS RN: 133745-75-2 | Numéro de produit: F0335
N-Fluorobenzenesulfonimide [Fluorinating Reagent]
![N-Fluorobenzenesulfonimide [Fluorinating Reagent] No-Image](/medias/F0335.jpg?context=bWFzdGVyfHJvb3R8NDYzMDV8aW1hZ2UvanBlZ3xhREZrTDJnMFlTODRPVEl4TmpnNU16VTRNelkyTDBZd016TTFMbXB3Wnd8NDJkMWVkY2MxYjM2N2FjOWNlNDk2ZjkxOWJhNjljYWEwNmQzMzY5ZTBhYWFlMDYyOGMxM2M5NmIzNjA3MmVhOQ)
Pureté: >98.0%(T)(HPLC)
- N-Fluorobis(phenylsulfonyl)amine
- NFSI
- NFBS
| Taille | Prix unitaire | Belgique | Japon * | Quantité |
|---|---|---|---|---|
| 5G |
€42.00
|
8 | ≥100 |
|
| 25G |
€163.00
|
2 | ≥100 |
|
*Le délai de livraison pour des produits disponibles en stock en Belgique est 1 à 2 jours
*Le délai de livraison pour des produits disponibles en stock en Japon est 1 à 2 semaines (sauf des produits réglementés et des envois avec de la glace carbonique)
| Numéro de produit | F0335 |
| Pureté / Méthode d'analyse | >98.0%(T)(HPLC) |
| Formule moléculaire / poids moléculaire | C__1__2H__1__0FNO__4S__2 = 315.33 |
| Etat physique (20 ° C) | Solid |
| Condition de stockage | Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 133745-75-2 |
| Numéro de registre de Reaxys | 5348902 |
| Identifiant de la substance PubChem | 87570107 |
| SDBS | 19601 |
| Numéro MDL | MFCD00144885 |
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Purity(Iodometric Titration) | min. 98.0 % |
| Melting point | 115.0 to 119.0 °C |
| Point de fusion | 115 °C |
| Pictogramme |


|
| Mot de signal | Attention |
| Mentions de danger | H315 : Provoque une irritation cutanée. H319 : Provoque une sévère irritation des yeux. H341 : Susceptible d'induire des anomalies génétiques. |
| Conseils de prudence | P501 : Éliminer le contenu/récipient dans une installation d'élimination des déchets agréée. P201 : Se procurer les instructions spéciales avant utilisation. P264 : Se laver la peau soigneusement après manipulation. P280 : Porter des gants de protection/ des vêtements de protection/ un équipment de protection des yeux/ du visage/ auditive. P308 + P313 : EN CAS d'exposition prouvée ou suspectée: consulter un médecin. P337 + P313 : Si l'irritation oculaire persiste: consulter un médecin. |
| N ° SH (import / export) (TCI-E) | 2935909099 |

-
Used Chemicals
-
Procedure
-
To a solution of 4-bromobiphenyl (1.17 g, 5.00 mmol), DABSO (721 mg, 3.00 mmol), and PdCl2(Amphos)2 (177 mg, 0.25 mmol) in anhydrous isopropyl alcohol (20 mL) was added triethylamine (2.1 mL, 15 mmol) at room temperature and the mixture was stirred at 75 °C for 23 hours. The suspension was cooled at room temperature and NFSI (2.36 g, 7.5 mmol) was added and the reaction mixture stirred for 3 hours until completion. The solvent was removed under reduced pressure. The residue was diluted with ethyl acetate (20 mL) and filtrated through celite pad. The filtrate was washed with saturated aqueous Na2S2O3 solution (20 mL) and brine (20 mL), dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by medium pressure preparative chromatography (ethyl acetate:hexane = 2:98 - 5:95 on silica gel) to give compound 1 as a white solid (928 mg, 79%).
-
Experimenter’s Comments
-
The reaction mixture was monitored by TLC (ethyl acetate:hexane = 1:9, Rf = 0.57) and UPLC-MS.
-
Analytical Data
-
Compound 1
1H NMR (400 MHz, CDCl3); δ 8.12–8.04 (m, 2H), 7.86–7.80 (m, 2H), 7.67–7.58 (m, 2H), 7.44-7.55 (m, 3H).
-
Lead Reference
-
- One-pot palladium-catalyzed synthesis of sulfonyl fluorides from aryl bromides
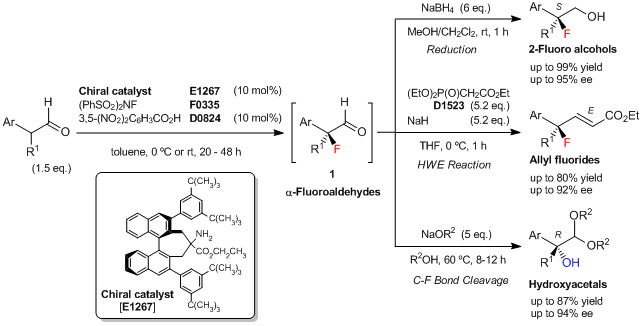
To a solution of the chiral amine catalyst [E1267] (20 mg, 0.026 mmol, 10 mol%) in toluene (0.54 mL) is added 3,5-dinitrobenzoic acid (5.5 mg, 0.026 mmol, 10 mol%), 2-phenylpropionaldehyde (52 mg, 0.39 mmol, 1.5 eq.), and N-fluorobenzenesulfonimide (NFSI) (0.26 mmol, 82 mg, 1 eq.) at 0 °C. The reaction mixture is stirred at 0 °C for 48 h, then poured into CH3OH/CH2Cl2 (1:4, 1.3 mL) at 0 °C. To this solution, NaBH4 (1.6 mmol, 6 eq.) is added, and the mixture is stirred at room temperature for 1 h. The reaction is quenched with saturated aq.NH4Cl, and the mixture is extracted with Et2O. The organic layer is dried over Na2SO4, and concentrated. The residue is purified by silica gel chromatography (eluent: hexane/ethyl acetate = 3/1) to give (S)-2-fluoro-2-phenylpropan-1-ol (34.5 mg, 86% yield based on NFSI, 95% ee) as a white solid.
References

In a dry 25 mL round-bottom flask, thiophene (0.5 mmol, 1 eq.), NFSI (0.6 mmol, 1.2 eq.) and CuI (5 mol%) are dissolved in DCE (3 mL), the resulting mixture is put into a preheated oil bath (60 °C) for 8 h. The solution is then cooled to room temperature, evaporated under reduced pressure, diluted with ethyl acetate, washed with a saturated aqueous solution of NaCl, dried over Na2SO4, filtered and evaporated under reduced pressure. The crude product is purified by silica gel column chromatography to afford the corresponding product (166.7 mg, Y. 88 %).
References

Reference
Articles / Brochures
[Research Articles] Copper-catalyzed Direct Amidation of Heterocycles with N-Fluorobenzenesulfonimide
[TCI Practical Example] One-Pot Palladium-Catalyzed Fluorosulfonylation of an Aryl Bromide Using DABSO and NFSI
Fiche de sécurité (FDS)
La FDS demandée n'est pas disponible.
Nous contacter pour plus d'informations.
Spécifications
CoA et autres Certificats
Exemple de CoA
Un échantillon CoA pour ce produit n'est pas disponible pour le moment.
Graphiques analytiques
Le tableau analytique demandé n'est pas disponible. Nous sommes désolés pour ce désagrément.



![N-Fluorobenzenesulfonimide [Fluorinating Reagent] N-Fluorobenzenesulfonimide [Fluorinating Reagent]](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)

