Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
CAS RN: 12148-71-9 | Numéro de produit: C2662
(1,5-Cyclooctadiene)(methoxy)iridium(I) Dimer

Pureté:
- Bis(1,5-cyclooctadiene)di-μ-methoxydiiridium(I)
| Taille | Prix unitaire | Belgique | Japon * | Quantité |
|---|---|---|---|---|
| 200MG |
€123.00
|
Contactez-nous | ≥40 |
|
| 1G |
€609.00
|
1 | ≥100 |
|
*Le délai de livraison pour des produits disponibles en stock en Belgique est 1 à 2 jours
*Le délai de livraison pour des produits disponibles en stock en Japon est 1 à 2 semaines (sauf des produits réglementés et des envois avec de la glace carbonique)
| Numéro de produit | C2662 |
| Formule moléculaire / poids moléculaire | C__1__8H__3__0Ir__2O__2 = 662.87 |
| Etat physique (20 ° C) | Solid |
| Condition de stockage | Frozen (<0°C) |
| Stocker sous gaz inerte | Store under inert gas |
| Condition à éviter | Light Sensitive,Air Sensitive,Moisture Sensitive,Heat Sensitive |
| Emballage Et Conteneur | 1G-Glass Bottle with Plastic Insert (Voir l'image) |
| CAS RN | 12148-71-9 |
| Numéro de registre de Reaxys | 14520157 |
| Identifiant de la substance PubChem | 160871404 |
| Numéro MDL | MFCD08459360 |
| Appearance | Light yellow to Amber to Dark green powder to crystal |
| Elemental analysis(Carbon) | 31.50 to 34.00 % |
| Point de fusion | 179 °C(dec.) |
| N ° SH (import / export) (TCI-E) | 2843909000 |
-
Used Chemicals
-
Procedure
-
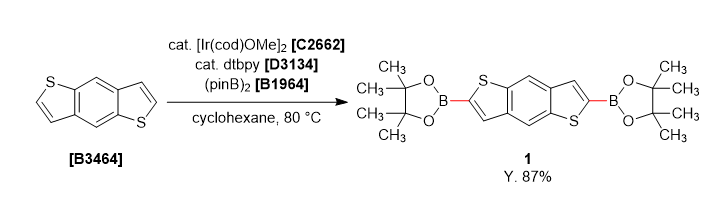
A solution of dtbpy (27 mg, 0.05 mmol), bis(pinacolato)diboron (1.0 g, 4.0 mmol) and [Ir(cod)OMe]2 (33 mg, 0.025 mmol) in cyclohexane (40 mL) was stirred under nitrogen at room temperature for 10 min. Benzo[1,2-b:4,5-b']dithiophene (380 mg, 2.0 mmol) was added the mixture and stirred 80 ˚C for 18 hours. The reaction mixture was quenched with water and separated both layers, extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate and filtered. The solvent was removed under reduced pressure and the crude was washed with methanol (20 mL) to give 1 as a white solid (0.771 g, 87% yield).
-
Experimenter’s Comments
-
The reaction mixture was monitored by NMR.
Cyclohexane was bubbled with nitrogen before use.
-
Analytical Data
-
Compound 1
1H NMR (270 MHz, CDCl3); δ 8.36 (s, 2H), 7.90 (s, 2H), 1.39 (s, 24H).
-
Lead Reference
-
- Synthesis and Transistor Application of Bis[1]benzothieno[6,7‑d:6′,7′‑d′]benzo[1,2‑b:4,5‑b′]dithiophenes

Reference
- Room temperature borylation of arenes and heteroarenes using stoichiometric amounts of pinacolborane catalyzed by iridium complexes in an inert solvent

An alcohol or ketone substrate is dissolved in THF and treated with a freshly prepared solution of [Ir(cod)OMe]2 (0.05 mol%) in THF and then with neat Et2SiH2 (1.2 eq.). The resulting solution is stirred at room temperature (23 °C) until complete conversion of the alcohol or ketone. At the completion of the reaction, the corresponding diethyl(hydrido)silyl ether is observed. Then the reaction mixture is placed under high vacuum for 1 h. The concentrated diethyl(hydrido)silyl ether is sequentially treated with freshly prepared solutions of norbornene (1.2 eq.) in THF and [Ir(cod)OMe]2 (0.5 mol%) in THF, and then with a slurry of Me4phen (1.25 mol%) in THF. The resulting solution is stirred at room temperature for 1 h and then heated it at 80-120 °C until complete conversion to the corresponding oxasilolane is observed. Then the crude reaction mixture containing the oxasilolane is sequentially treated with MeOH, KHCO3 (2.5 eq.) and H2O2 (30% solution in H2O, 10 eq.), and the resulting mixture is stirred overnight at 50 °C. The reaction is carefully quenched with aq. NaHSO3, and the resulting mixture is extracted with EtOAc. The combined organic layer is sequentially washed with 1 M HCl and sat. NaHCO3, and then dried with MgSO4. The resulting organic layer is filtered through Celite and concentrated to provide the crude diol, which is either purified directly or after conversion to the corresponding acetate derivative through treatment with Ac2O and Et3N.
References
Articles / Brochures
[Research Articles] Catalytic Functionalization of Un-activated Primary C-H Bonds
Fiche de sécurité (FDS)
La FDS demandée n'est pas disponible.
Nous contacter pour plus d'informations.
Spécifications
CoA et autres Certificats
Exemple de CoA
Un échantillon CoA pour ce produit n'est pas disponible pour le moment.
Graphiques analytiques
Le tableau analytique demandé n'est pas disponible. Nous sommes désolés pour ce désagrément.





