Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
CAS RN: 119752-83-9 | Numéro de produit: B3960
Bis(sulfur Dioxide)-1,4-diazabicyclo[2.2.2]octane Adduct
![Bis(sulfur Dioxide)-1,4-diazabicyclo[2.2.2]octane Adduct No-Image](/medias/B3960.jpg?context=bWFzdGVyfHJvb3R8NTkxMTF8aW1hZ2UvanBlZ3xhREZrTDJnMFlTODRPVEl3TVRnd016STJORE13TDBJek9UWXdMbXB3Wnd8MDlhZDVmMDUzNTU2OTI4OGU3MzVjYWFlOWYyYzcyNGYzYzI3NzM0NjQ2ODBhNDI3MzYzOWM3ZDRiZmEzZjRhNw)
Pureté: >94.0%(T)
- DABCO-Bis(sulfur Dioxide)
- DABSO
| Taille | Prix unitaire | Belgique | Japon * | Quantité |
|---|---|---|---|---|
| 1G |
€25.00
|
3 | ≥40 |
|
| 5G |
€91.00
|
4 | ≥80 |
|
*Le délai de livraison pour des produits disponibles en stock en Belgique est 1 à 2 jours
*Le délai de livraison pour des produits disponibles en stock en Japon est 1 à 2 semaines (sauf des produits réglementés et des envois avec de la glace carbonique)
| Numéro de produit | B3960 |
| Pureté / Méthode d'analyse | >94.0%(T) |
| Formule moléculaire / poids moléculaire | C__6H__1__2N__2O__4S__2 = 240.29 |
| Etat physique (20 ° C) | Solid |
| Condition de stockage | Refrigerated (0-10°C) |
| Stocker sous gaz inerte | Store under inert gas |
| Condition à éviter | Air Sensitive,Heat Sensitive |
| Emballage Et Conteneur | 1G-Glass Bottle with Plastic Insert (Voir l'image) |
| CAS RN | 119752-83-9 |
| Numéro de registre de Reaxys | 21034099 |
| Identifiant de la substance PubChem | 354335121 |
| Appearance | White to Almost white powder to crystal |
| Purity(Iodometric back titration) | min. 94.0 % |
| Point de fusion | 180 °C(dec.) |
| solubilité dans l'eau | Soluble |
| Pictogramme |

|
| Mot de signal | Attention |
| Mentions de danger | H315 : Provoque une irritation cutanée. H319 : Provoque une sévère irritation des yeux. |
| Conseils de prudence | P264 : Se laver la peau soigneusement après manipulation. P280 : Porter des gants de protection/ un équipement de protection des yeux/ du visage. P302 + P352 : EN CAS DE CONTACT AVEC LA PEAU: Laver abondamment à l’eau. P337 + P313 : Si l'irritation oculaire persiste: consulter un médecin. P362 + P364 : Enlever les vêtements contaminés et les laver avant réutilisation. P332 + P313 : En cas d'irritation cutanée: consulter un médecin. |
| N ° SH (import / export) (TCI-E) | 2933599590 |

-
Used Chemicals
-
- 4-Biphenylboronic Acid (contains varying amounts of Anhydride) [B2294]
- Nickel(II) Bromide Ethylene Glycol Dimethyl Ether Complex (=NiBr2(glyme)) [N1050]
- 3,4,7,8-Tetramethyl-1,10-phenanthroline (= tmphen) [T0847]
- Bis(sulfur Dioxide)-1,4-diazabicyclo[2.2.2]octane Adduct (= DABSO) [B3960]
- Lithium tert-Butoxide (= LiOtBu)
- tert-Butyl Bromoacetate [B1473]
- 1,3-Dimethyl-2-imidazolidinone (= DMI) [D1477]
-
Procedure
-
Under nitrogen atmosphere, a degassed DMI (20 mL) solution of 4-biphenylboronic acid (792 mg, 4.00 mmol), DABSO (577 mg, 2.40 mmol), tmphen (95 mg, 0.40 mmol), lithium tert-butoxide (320 mg, 4.00 mmol) and NiBr2(glyme) (123 mg, 0.40 mmol) was stirred at 100 °C for 14 hours. The mixture was cooled to room temperature and tert-butyl bromoacetate (1.17 mL, 8.00 mmol) was added and the reaction mixture stirred for 1 hour. Water (20 mL) was poured into the reaction mixture and the mixture was extracted with diethyl ether (50 mL x 3). The combined organic phase was washed with water (50 mL) and brine (40 mL), dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by medium pressure preparative chromatography (ethyl acetate:hexane = 5:95 - 15:85 on silica gel) to give compound 1 as a white solid (1.01 g, 76%).
-
Experimenter’s Comments
-
NiBr2(glyme) and LiOtBu were weighed into a sample tube in a glove box because these reagents are moisture sensitive. If these were used in air, it should be weighted quickly and used up the reagents as soon as possible after opening.
Since the ligand of NiBr2(glyme) may be desorbed under reduced pressure, depressurization operations is desirable to be finished as soon as possible.
The reaction mixture was monitored by TLC (ethyl acetate:hexane = 2:1, Rf = 0.38) and UPLC-MS.
-
Analytical Data
-
Compound 1
1H NMR (400 MHz, CDCl3); δ 8.00 (d, 2H, J = 8.9 Hz), 7.78 (m, 2H), 7.58–7.66 (m, 2H), 7.42-7.54 (m, 3H), 4.08 (s, 2H), 1.58 (s, 2H), 1.39 (s, 9H).
-
Lead Reference
-
- Nickel(II)-Catalyzed Synthesis of Sulfinates from Aryl and Heteroaryl Boronic Acids and the Sulfur Dioxide Surrogate DABSO

-
Used Chemicals
-
Procedure
-
To a solution of 4-bromobiphenyl (1.17 g, 5.00 mmol), DABSO (721 mg, 3.00 mmol), and PdCl2(Amphos)2 (177 mg, 0.25 mmol) in anhydrous isopropyl alcohol (20 mL) was added triethylamine (2.1 mL, 15 mmol) at room temperature and the mixture was stirred at 75 °C for 23 hours. The suspension was cooled at room temperature and NFSI (2.36 g, 7.5 mmol) was added and the reaction mixture stirred for 3 hours until completion. The solvent was removed under reduced pressure. The residue was diluted with ethyl acetate (20 mL) and filtrated through celite pad. The filtrate was washed with saturated aqueous Na2S2O3 solution (20 mL) and brine (20 mL), dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by medium pressure preparative chromatography (ethyl acetate:hexane = 2:98 - 5:95 on silica gel) to give compound 1 as a white solid (928 mg, 79%).
-
Experimenter’s Comments
-
The reaction mixture was monitored by TLC (ethyl acetate:hexane = 1:9, Rf = 0.57) and UPLC-MS.
-
Analytical Data
-
Compound 1
1H NMR (400 MHz, CDCl3); δ 8.12–8.04 (m, 2H), 7.86–7.80 (m, 2H), 7.67–7.58 (m, 2H), 7.44-7.55 (m, 3H).
-
Lead Reference
-
- One-pot palladium-catalyzed synthesis of sulfonyl fluorides from aryl bromides

Reference
- Copper(I)-catalyzed Sulfonylative Suzuki?Miyaura Cross-coupling
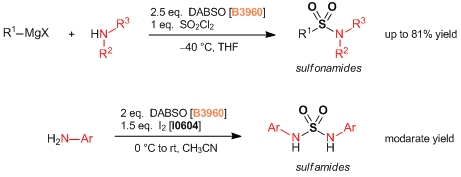
Reference
- DABCO- Bis (sulfur dioxide), DABSO, as a Convenient Source of Sulfur Dioxide for Organic Synthesis: Utility in Sulfonamide and Sulfamide Preparations
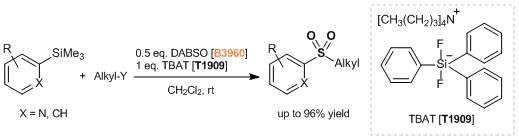
Reference
- Synthesis of Aromatic Sulfones from SO2 and Organosilanes Under Metal-free Conditions
Articles / Brochures
[Product Highlights] DABSO as a SO2 Source Usable for Direct Sulfonylative-Suzuki Coupling of Aryl Boronic Acids
[TCI Practical Example] One-Pot Palladium-Catalyzed Fluorosulfonylation of an Aryl Bromide Using DABSO and NFSI
Fiche de sécurité (FDS)
La FDS demandée n'est pas disponible.
Nous contacter pour plus d'informations.
Spécifications
CoA et autres Certificats
Exemple de CoA
Un échantillon CoA pour ce produit n'est pas disponible pour le moment.
Graphiques analytiques
Le tableau analytique demandé n'est pas disponible. Nous sommes désolés pour ce désagrément.



![Bis(sulfur Dioxide)-1,4-diazabicyclo[2.2.2]octane Adduct Bis(sulfur Dioxide)-1,4-diazabicyclo[2.2.2]octane Adduct](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)

