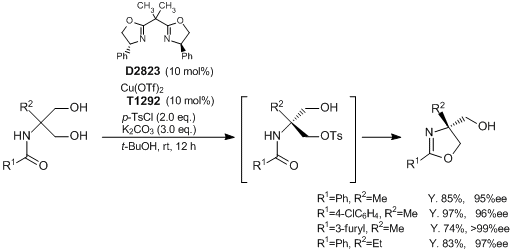
Onomura
et al. have reported the synthesis of optically active oxazolines through a copper-catalyzed asymmetric desymmetrization of 1,3-diols. According to their results, 2-(
N-acylamino)-1,3-propanediols react with
p-toluenesulfonyl chloride in the presence of
Cu(OTf)2 and
(R,R)-2,2'-isopropylidenebis(4-phenyl-2-oxazoline) as a catalyst to give the desired optically active oxazoline derivatives having a quaternary stereocenter in good to excellent yields with high enantioselectivities. The advantages of this method are a simple and easy operation and mild reaction conditions. Since this reaction system tolerates a diverse range of substrates, it is expected to be applied to the synthesis of various biologically active agents.