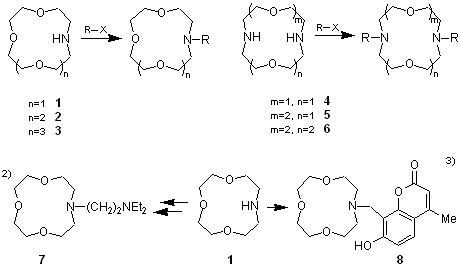
It is well known that crown ethers have good coordinating ability to metal ions. Armed crown ethers intoduce guest-ligating sidearms to the crown rings to form dynamic and three dimentional complexes which are attracting increased interest as intelligent molecules.1)
These compounds, 1-6, have nitrogen atoms at their crown rings, and via the introduction of sidearms by reaction with electrophiles, can be transformed in to armed crown ethers exhibiting new functionality. For example, 7 having an amine-functionalized sidearm shows high lithium ion selectivity,2) while it is formally size-fitted to the 12-crown 4-ether cavity which exhibits sodium ion selectivity.
Furthermore, armed crown ether 8, a metal-ligating sidearm which contains a chromophore, is used for the liquid-liquid extraction of alkali metal ions, and can monitor these ions by colorimetry.3)
Published TCIMAIL newest issue No.200