Maintenance Notice (4:30 AM - 11:00 AM December 21, 2024): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
| Einheit | Stückpreis | Belgien | Japan* | Menge |
|---|---|---|---|---|
| 1G |
86,00 €
|
Kontaktieren Sie uns | 14 |
|
| 10G |
264,00 €
|
1 | 8 |
|
*In Belgien verfügbare Lagerbestände werden in 1 bis 3 Tagen geliefert.
*In Japan verfügbare Lagerbestände werden in 1 bis 2 Wochen geliefert. (unter Ausschluss von regulierten Artikeln und Trockeneislieferungen).
| Artikel # | B6316 |
| Reinheit / Analysenmethode | >95.0%(T)(HPLC) |
| Summenformel / Molekülmasse | C__1__4H__1__9NO__6 = 297.31 |
| Physikalischer Zustand (20 °C) | Solid |
| Lagerungstemperatur | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Unter Inertgas lagern | Store under inert gas |
| Zu vermeidende Bedingungen | Air Sensitive |
| Verpackung und Behälter | 1G-Glass Bottle with Plastic Insert (Bild ansehen) |
| CAS RN | 19350-66-4 |
| Reaxys Registrierungsnummer | 489397 |
| PubChem-Stoff-ID | 468591034 |
| MDL-Nummer | MFCD00417306 |
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 95.0 area% |
| Purity(Neutralization titration) | min. 95.0 % |
| NMR | confirm to structure |
| Schmelzpunkt | 220 °C |
| Piktogramm |

|
| Signalwort | Achtung |
| Gefahrenhinweise | H315 : Verursacht Hautreizungen. H319 : Verursacht schwere Augenreizung. |
| Sicherheitshinweise | P264 : Nach Gebrauch Haut gründlich waschen. P280 : Schutzhandschuhe/ Augenschutz/ Gesichtsschutz tragen. P302 + P352 : BEI BERÜHRUNG MIT DER HAUT: Mit viel Wasser waschen. P337 + P313 : Bei anhaltender Augenreizung: Ärztlichen Rat einholen/ ärztliche Hilfe hinzuziehen. P362 + P364 : Kontaminierte Kleidung ausziehen und vor erneutem Tragen waschen. P332 + P313 : Bei Hautreizung: Ärztlichen Rat einholen/ ärztliche Hilfe hinzuziehen. |
| HS-Nr. (Import / Export) (TCI-E) | 2933399990 |
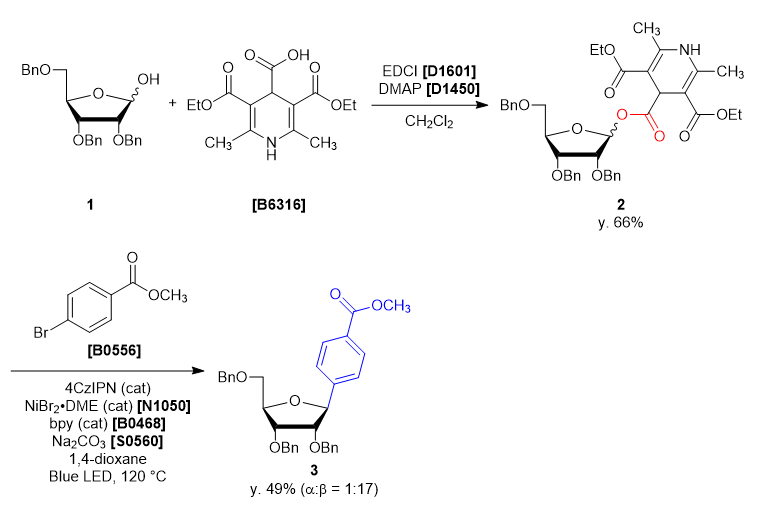
A four-neck round bottom flask was charged with 2,3,5-tri-O-benzyl-α/β-D-ribofuranose (1) (2.165 g, 5.15 mmol, 1 eq) and dichloromethane (26 mL). The solution was cooled under 5 ˚C, then DMAP (0.063 g, 0.51 mmol, 0.1 eq), EDCI (1.38 g, 7.21 mmol, 1.4 eq), 3,5-bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic acid (1.684 g, 5.66 mmol, 1.1 eq) was added. The reaction mixture was allowed to warm to ambient temperature and stirred for 46 h. After the reaction, the reaction mixture was quenched with ion-exchanged water (35 mL). The solution was transferred into a separatory funnel, and the aqueous layer was extracted with dichloromethane (10 mL, twice). The combined organic layers were washed with brine, dried over sodium sulfate (10 g) for about 30 minutes and then filtered. The solvent was removed in vacuo, giving crude as a blown oil (3.91 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 1:1, Rf = 0.45) to give compound 2 as a light yellow oil (2.99 g, y. 66%).
30 mL sealed vessel was charged with 4CzIPN (0.0158 g, 0.020 mmol, 0.1 eq), methyl 4-bromobenzoate (0.0858 g, 0.399 mmol, 2.0 eq), sodium carbonate (0.0443 g, 0.418 mmol, 2.1 eq), 2,2’-bipyridine (0.0086 g, 0.055 mmol, 0.28 eq) at rt under N2. A solution of NiBr2・DME (0.012 g, 0.040 mmol, 0.20 eq) in 1,4-dioxane (5 mL) and the solution of 1 (0.14 g, 0.20 mmol, 1.0 eq) in 1,4-dioxane (5 mL) were added to the sealed vessel. The mixture was placed in a preheated oil bath whose temperature was set to 120 ˚C, and placed at a distance of 2-3 cm from Blue LED lamp. After irradiation for 46 h, the reaction mixture was cooled to room temperature and filtered through Celite pad (1 cm). The solvent was removed in vacuo and the crude was given as a yellow oil (0.23 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 10:2, Rf = 0.30) to give compound 3 as a colorless oil (0.053 g, y. 49%).
Compound 3
1H NMR (270 MHz, CDCl3); δ 7.96 (d, J = 8.2 Hz, 2H), 7.46 (d, J = 8.2 Hz, 2H), 7.40-7.30 (m, 11H), 7.26-7.23 (m, 2H), 7.18-7.15 (m, 2H), 5.05 (d, J = 6.9 Hz, 1H), 4.71-4.44 (m, 6H), 4.39-4.34 (m, 1H), 4.01 (dd, J = 5.3, 3.3 Hz, 1H), 3.92 (s, 3H), 3.77 (dd, J = 6.9, 5.3 Hz, 1H), 3.65 (qd, J = 10.6, 3.9 Hz, 2H).
Das angeforderte SDB ist nicht verfügbar.
Bitte Kontaktieren Sie uns für mehr Informationen.
Ein Muster-AZ für dieses Produkt ist zur Zeit nicht verfügbar.
Das angeforderte Analysediagramm ist nicht verfügbar. Wir entschuldigen uns für die Unannehmlichkeiten.
