Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
CAS RN: 32005-36-0 | Produkte #: B1374
Bis(dibenzylideneacetone)palladium(0)

Reinheit:
- Palladium(0) Bis(dibenzylideneacetone)
- Bis(dba)palladium(0)
- Pd(dba)2
| Einheit | Stückpreis | Belgien | Japan* | Menge |
|---|---|---|---|---|
| 1G |
€116.00
|
14 | ≥100 |
|
| 5G |
€465.00
|
6 | ≥100 |
|
*In Belgien verfügbare Lagerbestände werden in 1 bis 3 Tagen geliefert.
*In Japan verfügbare Lagerbestände werden in 1 bis 2 Wochen geliefert. (unter Ausschluss von regulierten Artikeln und Trockeneislieferungen).
| Artikel # | B1374 |
| Summenformel / Molekülmasse | C__3__4H__2__8O__2Pd = 575.02 |
| Physikalischer Zustand (20 °C) | Solid |
| Lagerungstemperatur | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Unter Inertgas lagern | Store under inert gas |
| Zu vermeidende Bedingungen | Air Sensitive |
| Verpackung und Behälter | 1G-Glass Bottle with Plastic Insert (Bild ansehen) |
| CAS RN | 32005-36-0 |
| PubChem-Stoff-ID | 87564299 |
| Spektrale Daten (AIST) Link | 50493 |
| MDL-Nummer | MFCD00051942 |
| Appearance | Brown to Black powder to crystal |
| Content (Palladium) | 16.5 to 20.5 % |
| Schmelzpunkt | 150 °C |
| Piktogramm |

|
| Signalwort | Achtung |
| Gefahrenhinweise | H302 + H312 + H332 : Gesundheitsschädlich bei Verschlucken, Hautkontakt oder Einatmen. H315 : Verursacht Hautreizungen. H319 : Verursacht schwere Augenreizung. |
| Sicherheitshinweise | P261 : Einatmen von Staub vermeiden. P264 : Nach Gebrauch Haut gründlich waschen. P280 : Schutzhandschuhe/ Schutzkleidung/ Augenschutz/ Gesichtsschutz/ Gehörschutz Tragen. P337 + P313 : Bei anhaltender Augenreizung: Ärztlichen Rat einholen/ ärztliche Hilfe hinzuziehen. P302 + P352 + P312 : BEI BERÜHRUNG MIT DER HAUT: Mit viel Wasser waschen. Bei Unwohlsein GIFTINFORMATIONSZENTRUM/Arzt anrufen. P304 + P340 + P312 : BEI EINATMEN: Die Person an die frische Luft bringen und für ungehinderte Atmung sorgen. Bei Unwohlsein GIFTINFORMATIONSZENTRUM/Arzt anrufen. |
| HS-Nr. (Import / Export) (TCI-E) | 2843909000 |
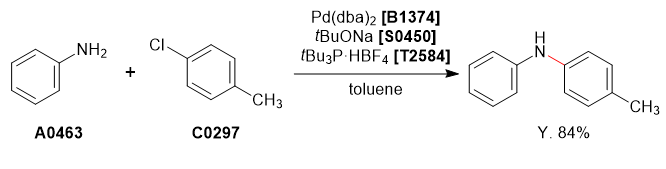
Used Chemicals
Procedure
Aniline (1.0 g, 10.7 mmol) and 4-chlorotoluene (1.4 g, 11.8 mmol) were dissolved in degassed toluene (10 mL) under nitrogen atmosphere. To this solution was added sodium tert-butoxide (1.54 g, 16.1 mmol), tri-tert-butylphosphonium tetrafluoroborate (31.1 mg, 0.1 mmol) and Pd(dba)2 (61.7 mg, 0.1 mmol, 1.5mol%). The reaction mixture was refluxed for 24 h and cooled to room temperature and quenched with H2O (10 mL). The organic phase was separated and washed with brine (10 mL), dried over MgSO4 (10 g), concentrated under reduced pressure to afford the crude product as a brown liquid. The crude product was purified by column chromatography on silica-gel (hexane : ethyl acetate = 95 : 5) to afford the desired product as a pale yellow crystalline solid (1.21 g, 6.6 mmol, 62%).
Experimenter’s Comments
Toluene was degassed by bubbling with N2 gas for 30 min.
The reaction mixture was analyzed by TLC (hexane : ethyl acetate = 1 : 1, Rf = 0.30) and GC.
Analytical Data (4-Methyl-N-phenylaniline)
1H NMR (400 MHz, CDCl3); δ 7.24-7.20 (t, J = 8.7 Hz, 3H), 7.07 (d, J = 8.7 Hz, 2H), 7.00-6.88 (m, 4H), 6.88-6.84 (m, 1H), 5.58 (s, 1H), 2.29 (s, 3H).
Lead Reference
- Tetramethoxybenzene is a Good Building Block for Molecular Wires: Insights from Photoinduced Electron Transfer
Used Chemicals
- 2-Chloro-m-xylene [C2164]
- 2-Methylphenylboronic Acid [M1313]
- Bis(dibenzylideneacetone)palladium (0) (Pd(dba)2) [B1374]
- 2-Dicyclohexylphosphino-2',6'-dimethoxybiphenyl (SPhos) [D5036]
- Tripotassium Phosphate (K3PO4)
- Toluene
- Ion-exchange water
Procedure
To a 3-necked flask was charged with bis(dibenzylideneacetone)palladium (0) (66 mg, 0.115 mmol, 1.5 mol%), SPhos (94 mg, 0.229 mmol, 3.0 mol%), 2-methylphenylboronic acid (1.56 g, 11.5 mmol, 1.5 equiv.), tripotassium phosphate (4.87 g, 22.9 mmol, 3.0 equiv.) degassed toluene (15 mL), and ion-exchange water (1.5 mL) under nitrogen atmosphere. The mixture was stirred at room temperature for 5 min. 2-Chloro-m-xylene (1.0 mL, 7.64 mmol, 1.0 equiv.) was added one portion. The resulting mixture was stirred at reflux for 7 h. The reaction mixture was cooled to room temperature and quenched with water (10 mL). The organic layer was washed with water (10 mL), and brine (10 mL), dried with Na2SO4 (20 g) and then concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (hexane only) to afford the corresponding compound as a colorless oil (1.46 g, 97%).
Experimenter's Comments
i) Toluene was degassed by bubbling with nitrogen gas for 30 min.
ii) The reaction mixture was monitored by GC.Analytical Data(2,2',6-Trimethyl-1,1'-biphenyl)
1H NMR (400 MHz, CDCl3); δ 7.23-7.33 (m, 3H), 7.22-7.10 (m, 3H), 7.00-7.06 (m, 1H), 1.99 (s, 3H), 1.97 (s, 6H).
13C NMR (101 MHz, CDCl3); δ 141.0, 140.5, 135.8, 135.5, 129.9, 128.8, 127.2 127.0, 126.9, 126.0, 20.3, 19.4.
Lead Reference
- Catalysts for Suzuki−Miyaura Coupling Processes: Scope and Studies of the Effect of Ligand Structure
Used Chemicals
Procedure
-
To a 2-necked flask was charged with bis(dibenzylideneacetone)palladium (0) (36 mg, 0.0633 mmol, 1.5 mol%), XPhos (60 mg, 0.127 mmol, 3.0 mol%), sodium tert-butoxide (811 mg, 8.44 mmol, 2.0 equiv.) and toluene (5 mL) under nitrogen atmosphere. The mixture was stirred at room temperature for 5 min. 4-chlorotoluene (0.5 mL, 4.22 mmol, 1.0 equiv.), and morpholine (0.55 mL, 6.33 mmol, 1.5 equiv.) were added in one portion. The resulting mixture was stirred at reflux for 6 h. The reaction mixture was cooled to room temperature and quenched with water (10 mL). The organic layer was washed with water (10 mL), and brine (10 mL), dried with Na2SO4 (20 g) and then concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (hexane : ethyl acetate = 9 : 1) to afford the corresponding compound as an orange solid (700 mg, 94%).
Experimenter's Comments
Toluene was degassed by bubbling with nitrogen gas for 30 min.
The reaction mixture was monitored by GC.Analytical Data(Compound 1)
1H NMR (400 MHz, CDCl3); δ 7.10 (d, J = 8.7 Hz, 2H), 6.84 (d, J = 8.7 Hz, 2H), 3.87 (t, J = 4.8 Hz, 4H), 3.11 (t, J = 4.8 Hz, 4H), 2.28 (s, 3H).
13C NMR (101 MHz, CDCl3); δ 149.1, 129.7, 129.5, 116.0, 66.9, 49.9, 20.4.
Lead Reference
- Expanding Pd-Catalyzed C−N Bond-Forming Processes: The First Amidation of Aryl Sulfonates, Aqueous Amination, and Complementarity with Cu-Catalyzed Reactions
- Pd-Catalyzed Kumada-Corriu Cross-Coupling Reactions at Low Temperatures Allow the Use of Knochel-type Grignard Reagents


References
Artikel / Broschüren
Sicherheitsdatenblatt (SDB)
Das angeforderte SDB ist nicht verfügbar.
Bitte Kontaktieren Sie uns für mehr Informationen.
Spezifikationsdokumenten
AZ & andere Zertifikate
Muster-AZ
Ein Muster-AZ für dieses Produkt ist zur Zeit nicht verfügbar.
Analytische Diagramme
Das angeforderte Analysediagramm ist nicht verfügbar. Wir entschuldigen uns für die Unannehmlichkeiten.







![(4,4'-Di-tert-butyl-2,2'-bipyridine)bis[3,5-difluoro-2-[5-trifluoromethyl-2-pyridinyl-κN)phenyl-κC]iridium(III) Hexafluorophosphate (4,4'-Di-tert-butyl-2,2'-bipyridine)bis[3,5-difluoro-2-[5-trifluoromethyl-2-pyridinyl-κN)phenyl-κC]iridium(III) Hexafluorophosphate](/medias/D5817.jpg?context=bWFzdGVyfHJvb3R8MjU2NzV8aW1hZ2UvanBlZ3xhRGxtTDJnMU5TODVNVEUyT0RJMk1qTTVNREEyTDBRMU9ERTNMbXB3Wnd8YmJkMWY4YTBjMWZkYTI1ZDRhZTVkNzRmZTk3YzM3N2FiZTYzNDUwNGJlNzEzMjJlODIzZDUyMzA0NWNiNThjMQ)

