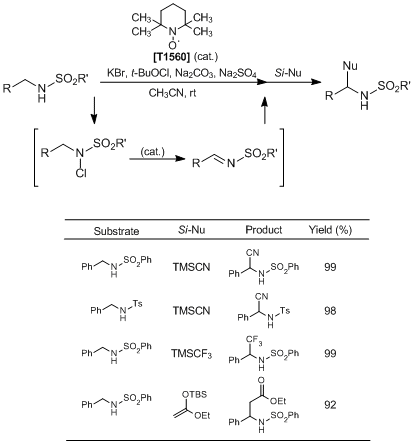
Moriyama
et al. have reported the nitroxyl-radical-catalyzed oxidative coupling of amides with silylated nucleophiles. According to their results, in situ generated
N-halogenated amides from amides and a halogenation reagent are activated by the treatment of a nitroxyl-radical
TEMPO catalyst to afford the corresponding imine intermediates, which further react with silylated nucleophiles giving the desired coupling products in high yields. This reaction opened up new frontiers in nitroxyl-radical-catalyzed reactions, so it is expected that nitroxyl radicals can be used as organocatalysts in various transformations.