盲盒好礼,精彩继续 | 轻松扫码查看产品文档 | TCIMAIL No.198 已上新 | TCI试剂——品质可靠,值得信赖
订购方法?联系方式:021-67121386 / Sales-CN@TCIchemicals.com
Maximum quantity allowed is 999
请选择数量
Sparteines as Chiral Ligands for Asymmetric Synthesis
No.159(October 2013)
- S0461
- (−)-Sparteine (1) (*This product is discontinued.)
- S0884
- (+)-Sparteine (2)
The alkaloid (−)-sparteine (1) has found widespread use as a chiral ligand for asymmetric
reactions.1) The complex formed from 1 and organolithium has recognized enantiotopic sides in
its carbanions and prochiral reaction partner’s protons.2,3) Asymmetric aldol additions using 1
and TiCl4 have provided aldol products with excellent chiral selectivities.4)
Palladium-catalyzed oxidation and the following resolutions of secondary alcohols assisted by 1 as a ligand
have provided the optically activated alcohols.5) In addition, 1 has been used for
enantiomer-selective polymerizations.6)
(+)-Sparteine (2) is not easily obtained from natural source compared with 1.
However, 2 has potential for a chiral ligand which affords the products having an opposite configuration to
those obtained by using 1.3)

References
- 1) Review of (−)-sparteine as a chiral ligand for metal catalysts
- 2) Review of enantioselective synthesis with Li/(−)-sparteine carbanion pairs
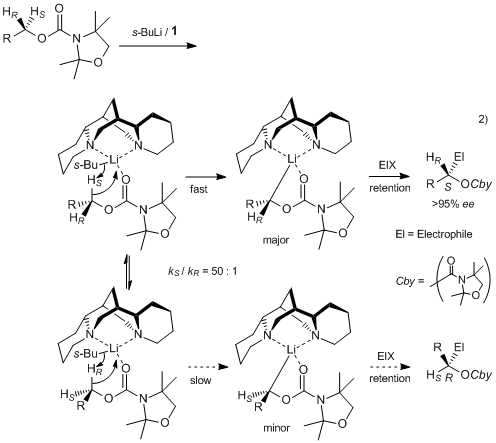
- 3) Asymmetric lithiation of (−)-sparteine and (+)-sparteine
- 4) Asymmetric aldol additions using (−)-sparteine and TiCl4
- 5) Palladium-catalyzed oxidative kinetic resolutions of secondary alcohols with (−)-sparteine
- 6) Enantiomer-selective polymerizations using (−)-sparteine
The prices are subject to change without notice. Please confirm the newest price by our online
catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.

