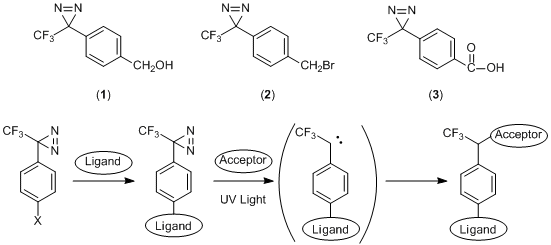
Cross-linkers Containing Photoreactive Diazirine Group
Diazirine changes to a high-reactive carbene by absorbing light near 360 nm, which forms a covalent bond to a nearby molecule. The covalent bond is more stable than one formed by nitrene derived from a photoreactive azide.
Phenyldiazirine derivatives 1–3 have a functional group at the para position. For example, after the functional group attaches to a ligand, the diazirine group reacts with the acceptor molecule under photoexcitation. It has been reported in research of the photoaffinity labelings which include the crosslinker with peptides or sugars,2) and the photoaffinity microarrays.3)
References
- 1)Reviews
- a)T. Tomohiro, M. Hashimoto, Y. Hatanaka, Chem. Record 2005, 5, 385.

- b)M. Hahimoto, Y. Hatanaka, Eur. J. Org. Chem. 2008, 2513.

- c)Y. Sadakane, YAKUGAKU ZASSHI 2007, 127, 1693.

- 2)Photoaffinity labeling
- a)Y. Kashiwayama, T. Tomohiro, K. Narita, M. Suzumura, T. Glumoff, J. K. Hiltunen, P. P. V. Veldhoven, Y. Hatanaka, T. Imanaka, J. Biol. Chem. 2010, 285, 26315.

- b)E. W. S. Chan, S. Chattopadhaya, R. C. Panicker, X. Huang, S. Q. Yao, J. Am. Chem. Soc. 2004, 126, 14435.

- c)K. Matsuda, M. Ihara, K. Nishimura, D. B. Sattelle, K. Komai, Biosci. Biotechnol. Biochem. 2001, 65, 1534.

- d)M. Wiegand, T. K. Lindhorst, Eur. J. Org. Chem. 2006, 4841.

- 3)Photoaffinity microarray
- a)D. M. Dankbar, G. Gauglitz, Anal. Bioanal. Chem. 2006, 386, 1967.

- b)S. Wei, J. Wang, D.-J. Guo, Y.-Q. Chen, S.-J. Xiao, Chem. Lett. 2006, 35, 1172.

- c)N. Kanoh, S. Kumashiro, S. Simizu, Y. Kondoh, S. Hatakeyama, H. Tashiro, H. Osada, Angew. Chem. Int. Ed. 2003, 42, 5584.

- 4)Phenyldiazirine Synthesis
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.