轻松扫码查看产品文档 | TCIMAIL No.197 已上新 | TCI试剂——品质可靠,值得信赖
订购方法?联系方式:021-67121386 / Sales-CN@TCIchemicals.com
Maximum quantity allowed is 999
请选择数量
Highly Potent Chiral Derivatizing Reagents
No.126(August 2007)
- A1657
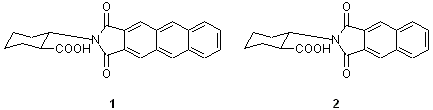
- (1R,2R)-2-(Anthracene-2,3-dicarboximido)cyclohexanecarboxylic Acid (1a)
- A1658
- (1S,2S)-2-(Anthracene-2,3-dicarboximido)cyclohexanecarboxylic Acid (1b)
- N0713
- (1R,2R)-2-(Naphthalene-2,3-dicarboximido)cyclohexanecarboxylic Acid (2a)
- N0714
- (1S,2S)-2-(Naphthalene-2,3-dicarboximido)cyclohexanecarboxylic Acid (2b)
In the recent development in pharmaceuticals, agrochemicals and functional materials including liquid crystals, the importance of chiral compounds is increasingly emphasized, and discrimination of enantiomers is considered to be a more and more important subjects. One of the most common enantiomeric discrimination methods utilizes diastereomer which are obtained from the reaction of the enantiomers and chiral derivatizing reagents, and many excellent chiral derivatizing reagents have been developed. This method utilizing chiral derivatizing reagents, however, has a problem in that it is very difficult to discriminate derivatized diastereomers having chiral centers more than four carbon atoms away from derivatization site.
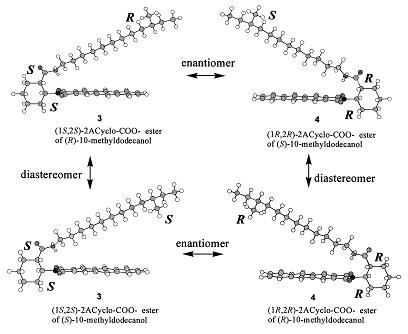
Fig. Configuration model, 1 ester of 10-methyldodecanol.
1a: (1R,2R)-2ACyclo-COO-, 1b: (1S,2S)-2ACyclo-COO-.
1a: (1R,2R)-2ACyclo-COO-, 1b: (1S,2S)-2ACyclo-COO-.
Recently, Ohrui and co-workers have developed highly sensitive chiral derivatizing reagents 1 and 2 that overcome this problem, and have reported on their efficiency. For instance, 1 reacts with a long-chain alcohol to form esters 3 and 4, where the aromatic ring of the chiral derivatizing reagent and the methylene chain of the alcohol are in a gauche configuration. Thus, these esters exhibit only one helical structure (clockwise or counterclockwise) based on the absolute configuration of the chiral derivatizing reagents. In cases where a long chain alcohol have a chirality, the esters 3 and 4 has both an asymmetric helical configuration and a chiral center. Moreover, if this alcohol is racemic form, it forms diastereo-isomers. These diasteromers can be discriminated by HPLC and NMR.
1 and 2 can be easily discriminated when their chiral centers are far away from the hydroxyl group of an alcohol. However, in HPLC analysis, 1 is effective for the discrimination of an chiral center more than 11 carbon atoms away from the hydroxyl group, while 2 is effective for discrimination of an chiral center less than 10 carbon atoms away from the hydroxyl group. In particular, since 1 possesses potent fluorescence derived from its anthracene moiety, determination can be done at a femto molar (10-15 M) level. Moreover, these reagents can discriminate the chirality of a secondary hydroxyl group, thus, it is also possible to separate steroisomers of branched secondary alcohols by HPLC.
Mori and co-workers have determined the optical purity of synthetic intermediate (6S,19S)-6-hydroxy-19-methylnonaicosadecane in the synthesis of a sex pheromone extracted from female Spiral Uji fly (Screwworm fly) using reagent 1. The reagents 1 and 2 developed by Ohrui and co-wokers can be discriminated of chiral centers far away from hydroxyl groups, which used to be impossible by the diastereomer methods. Thus, these reagents are highly expected to be useful for many applications in practical use including determination of the absolute configuration and optical purity of natural products.
References
- 1)Highly potent chiral derivatizing reagents
- H. Ohrui, H. Terashima, K. Imaizumi, K. Akasaka, Proc. Jpn. Acad., Ser. B: Phys. Biol. Sci. 2002, 78B, 69.
- K. Imaizumi, H. Terasima, K. Akasaka, H. Ohrui, Anal. Sci. 2003, 19, 1243.

- K. Mori, T. Ohtaki, H. Ohrui, D. R. Berkebile, D. A. Carlson, Eur. J. Org. Chem. 2004, 1089.

- T. Ohtaki, K. Akasaka, C. Kabuto, H. Ohrui, Chirality 2005, 17, S171.

- H. Ohrui, K. Akasaka, K. Imaizumi Kagaku To Seibutsu 2003, 41, 691.
- H. Ohrui, Bunseki Kagaku 2004, 53, 805.

The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.

