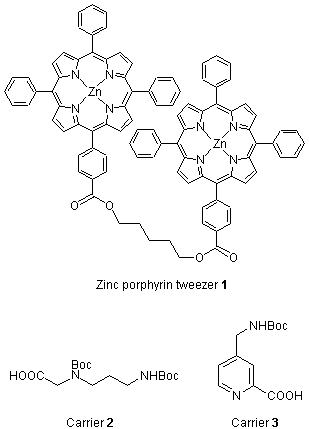
Circular dichroic (CD) exciton chirality method has been extensively utilized for non-empirical assignments of absolute configurations of a wide variety of compounds.1 However, since exciton coupling requires two or more interacting chromophores in the same molecular, the method cannot be applied in a straightforward manner to molecules which have only a single site for derivatization. Recently, the achiral bis-zinc porphyrin host 1 has been developed for absolute configurational assignment of chiral diamines from the induced CD of the complex formed from the achiral host 1 and a chiral diamine guest.2 This new approach has recently been extended for determining absolute configurations of two important types of chiral compounds, namely, monoalcohols and monoamines by the exciton chirality method.
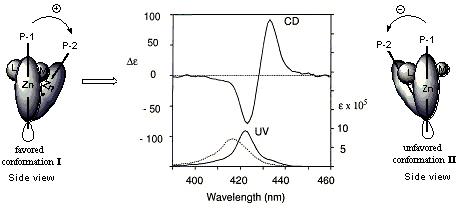
A monoalcohol, e.g., (+) isomenthol, is derivatized with the trifunctional carrier 2 to generate a bidentate ligand 4, which is capable of forming the 1 : 1 host / guest complex 5 with the chromophoric host zinc porphyrin tweezer 1.3,4 The porphyrin closer to the chiral center (P-2) in the complex 5 will prefer approaching the chiral center from the side of the M (medium) group in order to avoid the steric interference of the L (large) group. The absolute stereochemistry of the monoalcohol can then be easily determined from the sign of the exciton couplet of the complex 5, i.e., a positive CD couplet indicates a clockwise arrangement of the L, M, and S (large, medium, small) groups in the Newman projection of the monoalcohol with the hydroxyl group in the rear, and vice versa. The absolute configuration of monoamines can be determined in a similar manner as with monoalcohols utilizing carrier 3.5 The assignments of L, M, S groups are mostly based on conformational energies (A values)6, while in some cases, electronic factors and hydrogen bonding have to be considered.3,4
This sensitive method is applicable to monoalcohols and monoamines containing various side chains, such as aliphatic, aromatic, ester, amide and hydroxyl moieties and can be preformed at the several microgram level.
新客好礼,乐享不停 | 盲盒好礼,精彩继续 | 轻松扫码查看产品文档 | TCIMAIL No.199 已上新 | TCI试剂——品质可靠,值得信赖
订购方法?联系方式:021-67121386 / Sales-CN@TCIchemicals.com