新客好礼,乐享不停 |盲盒好礼,精彩继续 | 轻松扫码查看产品文档 | TCIMAIL No. 200 已上新 | TCI试剂——品质可靠,值得信赖
订购方法?联系方式:021-67121386 / Sales-CN@TCIchemicals.com
Maximum quantity allowed is 999

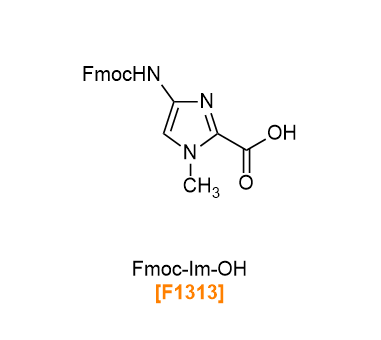
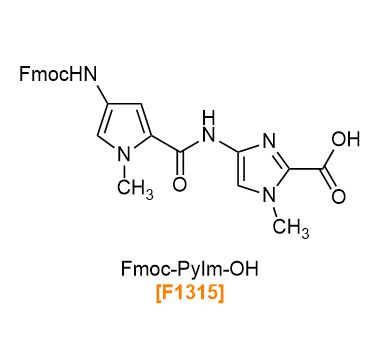
PIPs (pyrrole-imidazole (Py-Im) polyamides) are polyamides with 1-methylpyrrole (Py) and 1-methylimidazole (Im) as units. Within PIPs, amino and carbonyl groups sides are known to form Py/Py or Py/Im pairs, in which the amino and carbonyl groups face each other in antiparallel. PIPs recognize specific DNA base pairs via hydrogen bonds, enter the minor-groove of the DNA double helix to bind sequence-selectively. By changing the sequence of Py and Im, it is possible to design and bind to any DNA base sequences.1-4) We have Boc-Im-OH, Fmoc-Im-OH, Boc-Py-OH and Fmoc-Py-OH, amino groups of which are protected by Boc or Fmoc, as raw materials for the synthesis of PIPs. In addition, the PyIm block Fmoc-PyIm-OH, which is difficult to lengthen by solid-phase synthesis,5-7) is also available.
TCI can provide the analogs and the derivatives, as well as offers these raw materials at large scale.

Products
References
- 1) Recognition of DNA by designed ligands at subnanomolar concentrations
- 2) Extension of Sequence-Specific Recognition in the Minor Groove of DNA by Pyrrole−Imidazole Polyamides to 9−13 Base Pairs
- 3) Solid Phase Synthesis of Polyamides Containing Imidazole and Pyrrole Amino Acids
- 4) Chemical Approaches to the Development of Artificial Transcription Factors Based on Pyrrole-Imidazole Polyamides
- 5) Rapid solid-Phase synthesis of DNA-Binding pyrrole-Imidazole polyamides
- 6) Highly Efficient Synthesis of DNA-Binding Hairpin Polyamides via the Use of a New Triphosgene Coupling Strategy
- 7) Highly Efficient Synthesis of DNA-Binding Hairpin Polyamides via the Use of a New Triphosgene Coupling Strategy