Maximum quantity allowed is 999
Carbohydrate-binding Protein Related Reagents: Recombinant Lectins
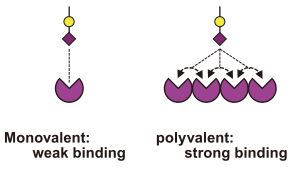
Lectins are highly specific carbohydrate-binding proteins of nonimmune origin. Due to their ability to bind with cell-surface glycoproteins and glycolipids, lectins could agglutinate cells; they also could reversibly associate polysaccharides and glycoproteins in solution.
Lectin has long been well-known as tools for detection and analysis of functional oligosaccharides in the glycoscience field. Recombinant lectins show better stability compared with lectins extracted from natural resources. TCI offers not only recombinant lectins but also chemically modified lectins such as several types of biotinylated lectin and lectin-agarose for detection and capturing of glycoconjugates.
LecBeads (Lectin-Agarose)
[Capturing for glycoprotein]

Products
Lectin-Biotin Conjugates
[Detection for glycoprotein, glycolipid]

Products
Lectins
[Multivalent effect by lectin]
Products
- R0225
- Recombinant Polyporus squamosus lectin (= rPSL1a) expressed in Escherichia coli
- R0226
- Recombinant Laetiporus sulphureus lectin N-Terminal Domain (= rLSL-N) expressed in Escherichia coli
- R0227
- Recombinant Marasmius oreades agglutinin (= rMOA) expressed in Escherichia coli
- R0228
- Recombinant Sclerotium rolfsii lectin (= rSRL) expressed in Escherichia coli
- R0229
- Recombinant Griffithsia sp. lectin (= rGRFT) expressed in Escherichia coli
- L0169
- Lectin, Fucose specific (= AOL) from Aspergillus oryzae
LecBeads for Capturing of Glycoconjugates (Recombinant Lectin-Agarose)
[Ex. Capturing for glycoproteins]

rPSL1a-LecBeads [R0235], rLSL-N-LecBeads [R0236], rMOA-LecBeads [R0237], rSRL-LecBeads [R0238], rGRFT-LecBeads [R0239] were developed in collaboration with National Institute of Advanced Industrial Science and Technology (AIST).
Recombinant Lectins for Lectin-sandwich ELISA and Hemagglutinin assay
[Ex. Carbohydrate-binding Specificity of Lectins]
Recombinant Polyporus squamosus lectin (= rPSL1a) expressed in Escherichia coli [R0225]
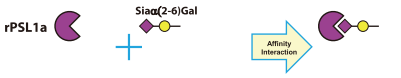
- Cloning, expression in Escherichia coli and characterization of the recombinant Neu5Acα2,6Galβ1,4GlcNAc-specific high-affinity lectin and its mutants from the mushroom Polyporus squamosus
- Structure and binding analysis of Polyporus squamosus lectin in complex with the Neu5Ac{α}2-6Gal{β}1-4GlcNAc human-type influenza receptor
Recombinant Laetiporus sulphureus lectin N-Terminal Domain (= rLSL-N) expressed in Escherichia coli [R0226]

- Structural analysis of the Laetiporus sulphureus hemolytic pore-forming lectin in complex with sugars
Recombinant Marasmius oreades agglutinin (= rMOA) expressed in Escherichia coli [R0227]
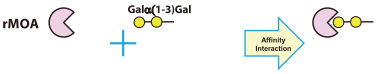
- Cloning, expression, and characterization of the Galα1,3Gal high affinity lectin from the mushroom Marasmius oreades
- Structural characterization of a lectin from the mushroom Marasmius oreades in complex with the blood group B trisaccharide and calcium
Recombinant Sclerotium rolfsiilectin (= rSRL) expressed in Escherichia coli [R0228]

- Carbohydrate specificity of a lectin isolated from the fungus Sclerotium rolfsii
- Structural basis for the carbohydrate recognition of the Sclerotium rolfsiilectin
Recombinant Griffithsiasp. lectin (= rGRFT) expressed in Escherichia coli [R0229]

- Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp
- Crystallographic studies of the complexes of antiviral protein griffithsinwith glucose and N-acetylglucosamine
Lectin, Fucose specific (= AOL) from Aspergillus oryzae (5mg/mL, PBS pH6.5) [L0169]

- Molecular cloning and overexpression of fleA gene encoding a fucose-specific lectin of Aspergillus oryzae,
- Carbohydrate binding specificity of a fucose-specific lectin from Aspergillus oryzae: a novel probe for core fucose,
rPSL1a [R0225], rLSL-N [R0226], rMOA [R0227], rSRL [R0228], rGRFT [R0229] were commercialized under license from National Institute of Advanced Industrial Science and Technology (AIST).
AOL[L0169] was merchandized under the technical tie-up with GEKKEIKAN SAKE COMPANY, LTD.
Biotinylated Recombinant Lectins for Lectin-staining and Lectin-blotting
[Ex. Detection for glycoproteins]


